
EVERSANA Team
EVERSANA employs a team of over 6000 professionals across 20+ locations around the world. From industry-leading patient service and adherence support to global pricing and revenue management, our team informs the strategies that matter the most to our clients and perform superior services that create value across the product life cycle.
Articles by EVERSANA Team

Navigating Successful Partnerships in the Pharmaceutical Industry: Insights from Shorla Oncology CEO Sharon Cunningham
In the ever-evolving landscape of the pharmaceutical industry, forging successful partnerships and collaborations is a cornerstone of achieving groundbreaking advancements and bringing innovative treatments to patients in need. However, navigating through the myriad of advisors, consultants and potential collaborators can be daunting for even the most seasoned professionals. Enter Sharon Cunningham, CEO and Co-Founder of […]

Measuring Willingness to Pay: The Next Access Frontier
EVERSANA’s APAC Team Authors: Robin Arnold, Swapnil Waichale, Lakshmi Pragna Kalavacharla, Utkarsh Sahu Patient access to expensive healthcare poses a significant challenge in the pharmaceutical industry. As the cost of developing innovative medicines rises, the demand for accessibility as a basic human right increases. This POV explores the evolution of Patient Access Programs (PAPs) from […]

CES 2024 Recap – How the Future of Healthcare Will Continue to Be Driven By Innovation & AI
CES 2024, the annual tech conference encompassing everything from cutting-edge gadgets to automotive marvels, healthcare innovations, and futuristic media, left a lasting impression. Amidst the buzz surrounding electric vehicles, transparent screens, and an abundance of massage chairs (so many massage chairs), there was one technology that overlapped across all: AI. The rapid integration of AI […]

Impact of the European Union Medical Device Regulation Extension on Legacy Devices
Question: The European Union (EU) has extended the transition period for medical devices under the Medical Device Regulation (MDR) and removed the sell-off provisions for existing products. How do these changes impact you? Answer: On 7 March 2023, the European Council voted to adopt a measure to extend the transitional period for medical devices under […]

The Hospital of the Future and How Data, Digital and AI Merge to Make Care Better
By Pierantonio Russo, MD, FCPP, FAAP, STS, Chief Medical Officer, EVERSANA In the fast-paced realm of healthcare, innovation stands as the cornerstone of progress. Recently, I had the privilege to attend Frontier’s Health , a leading gathering of some of the best minds in the world to discuss how to shape the future of health […]

Robotics in Healthcare: Revolutionizing Patient Care
Authors: Robin Arnold, Ankit Kulshrestha, Abheek Bose, Mayur Muley, Anant Kasodekar The launch of the PUMA 650 surgical robot in the 1980s, initially employed in brain biopsy procedures, ushered in the era of medical robots.1 From then until the present day, remarkable innovations have led to the development of state-of-the-art robots and their seamless integration […]

The Data-Driven Revolution: AI’s Impact on Pharmaceutical Quality in Digital Therapy
In the intricate web of pharmaceuticals, a groundbreaking transformation is underway – one that marries data analytics with digital therapy, reshaping the industry’s landscape fundamentally. As the dust settles from the FDA Symposium, a seismic shift in focus becomes apparent: the integration of Artificial Intelligence (AI) in Digital Therapy isn’t just a trend; it’s a […]

Navigating the Evolving Landscape of Internet Healthcare in China: Development Status, Challenges, and Future Prospects
Authors: Robin Arnold, Executive Vice President; Renyang Liu, Engagement Manager; Erica Wang, Associate Consultant Background Internet healthcare means using the internet to provide health and medical services. China has implemented “Internet Plus Healthcare” as a national policy, as a part of reforming the healthcare system. Internet healthcare in China is estimated at 150 billion RMB […]

Unveiling the Future: AI Opportunities in FDA Compliance, Insights from the OPQ Symposium
In the dynamic realm of pharmaceuticals, staying ahead of the curve is not just an advantage; it’s a necessity. Recently, our team had the privilege of attending the CDER’s Office of Pharmaceutical Quality (OPQ) symposium, where the focus was on a topic that’s transforming the industry: Artificial Intelligence (AI). As consultants deeply involved in FDA […]

Driving Patient-First Affordability in Seconds – Not Days: Accelerating Time to Therapy
Fewer than 60% of Pharmacy Benefit Manager (PBM) electronic benefit verifications (eBVs) accurately assess patients’ financial responsibilities, largely due to the industry’s reliance on AI algorithms for estimations. This leads to financial barriers for patients, hesitancy among healthcare providers (HCPs) to prescribe therapy, and increased burdens on Patient Assistance Programs (PAP) and Hubs. In this […]

Making Primary Market Research SMARTER Through Social Listening
Discover how EVERSANA is transforming the landscape of primary market research in the pharmaceutical industry through our unique approach to social listening. This insightful article from our APAC team highlights the common challenges faced by pharma companies and how most fall short in translating social insights into actionable strategies. EVERSANA, on the other hand, stands […]

APACMed Digital Health Reimbursement Policy Forum
Introduction The APACMed Policy Forum on Digital Health Reimbursement was held on 23 May 2023, with participants from Australia, Japan, Singapore, South Korea, Germany, France, and the UK. Several digital health policymakers, academicians, and experts from the respective countries, along with the APACMed Digital Health Reimbursement Alliance (DHRA) core committee, local trade associations, and EVERSANA […]

New Models of Benefits Verification Solve for Complexity, Accelerate Speed to Therapy and Deliver Price Transparency
Navigating health care insurance coverage can be a supremely convoluted process for anyone, especially patients or caregivers recently faced with coping with a life-threatening disease. While the advancement of many new treatments and scientific innovations in the form of “specialty drugs” has been invaluable in treating complex diseases, these conditions often muddy the benefit verification […]
Unlocking Global Success: The Crucial Role of Integrated HEOR Programs in New Product Launches
Author: Kirk Szafranski, Director, HEOR, Value & Evidence Health Economics and Outcomes Research (HEOR) programs play a pivotal role in assessing a product’s value, cost-effectiveness, and real-world impact on patient outcomes, which are critical factors influencing reimbursement decisions, formulary placements, and overall market access. A global integrated HEOR program typically includes a range of components […]

The Rising Demand for Light Medical Aesthetic in China
“The Rising Demand for Light Medical Aesthetic in China” provides a captivating insight into the surging popularity of non-surgical medical aesthetics in China. The article highlights the rapid growth of the medical aesthetic market and the shifting trends in consumer preferences. It delves into the key contributors and experts driving this industry and examines the […]

Revolutionizing Vaccines: The Rise of mRNA Technology in China
Key Contributors: Robin Arnold, Executive Vice President; Renyang Liu, Engagement Manager; Nicole Li, Analyst Discover how mRNA technology is transforming the field of vaccines in China. From the evolution of vaccine development to the rapid progress of mRNA vaccines during the COVID-19 pandemic, this article explores the advantages of mRNA vaccines compared to conventional ones. […]

Maximizing Potential: Essential Steps for Successful Commercialization of Cell and Gene Therapies
Introduction Cell and gene therapy (CGT) products have significantly enhanced the quality of life for millions of patients impacted by medical conditions that are untreatable with traditional medicines or surgeries such as certain cancers, inherited and rare diseases, and intractable conditions. As these therapies continue to propel groundbreaking advancements in remedying previously incurable diseases, the […]

The Art of Simplifying Pharmacovigilance – Part I: Connecting the Dots
The Art of Simplifying Pharmacovigilance – Part I: Connecting the Dots In the intricate realm of pharmacovigilance, there is an art — a delicate dance of connecting the dots. Like a skilled painter, vigilant professionals adeptly simplify the complex web of drug safety, linking scattered points to reveal a cohesive picture. With unwavering focus, they […]

Commercial Key Success Factors (KSFs) for Global Drug Development Programs
Key Contributors: Swapnil Waichale, Principal and Robin Arnold, Executive Vice President Discover the essential commercial key success Factors (KSFs) that drive triumph in global drug development programs. In an era of diverse regulatory perspectives and reimbursement complexities, biotech companies face higher stakes than ever before. Swapnil Waichale and Robin Arnold unveil the top failure modes […]

The Role of RWE in Expediting the Drug Approval Process
Key Contributors: Lydia Edison (Project Manager), Rohit Mandlik (Project Manager), Rohit Dang (Engagement Manager), Mahendra Rai (Senior Director) The role of real-world evidence (RWE) in expediting the drug approval process. RWE refers to the use of real-world data (RWD) from sources outside traditional clinical trials to support regulatory decision-making. RWE offers several benefits, including generating […]

Specialty Pharmacies are Ready for the Biosimilar Boom. Are you?
The pharmacy benefit biologic market is primed for disruption, with Specialty Pharmacies (SPs) eagerly awaiting the pending market shift. While biosimilars have been available for nearly a decade in the United States, this year will be the first time many SPs begin dispensing these brands. Currently, 22 biosimilars are available in the U.S. and, up […]

Position Your Future-Facing Strategy on Digital Therapeutics (DTx) in China
How can DTx resonate with current portfolios and capture incoming opportunities? Key Contributors: Robin Arnold, Executive Vice President; Jerry Song, Associate Principal; Qiwei (Alex) Li, Consultant The article discusses the potential of digital therapeutics (DTx) in China’s healthcare system and how it can help pharmaceutical and medical technology companies address the challenges presented by the country’s aging […]

A Comparison of Existing Real World Evidence (RWE) Guidelines
Key Contributors: Neel Patel, Associate Consultant- HEOR, APAC and Rajanikanth Manupati, Associate Consultant- HEOR, APAC In the fast-paced world of healthcare, where the development and approval of new therapies can take over a decade, the need for modern solutions is more pressing than ever. Enter Real World Evidence (RWE) – a game-changing approach that harnesses […]

Navigating the Complexities: Strategies for Asian Pharma Companies Entering the U.S. Market
Key Contributors: Divayum Gupta (Analyst), Tanay Sharma (Consultant), Sowbhagya Suresh (Senior Consultant), Jinsol Kim (Engagement Manager) The United States holds a dominant position in the global pharmaceutical market, accounting for 45% of global sales in 2022. However, Asian pharmaceutical companies face significant challenges when entering the complex U.S. market. The U.S. healthcare system involves multiple […]

Forecasting in the Age of Value-Based Agreements
The pharmaceutical industry faces a host of increasingly complex challenges and critical decisions when attempting to manage and predict their products’ plausible revenue patterns. The mishandling of revenue forecasting and evaluation can result in substantial financial liabilities, which has become more of an issue for manufacturers as products, disease states and additional factors that previously […]

Key Considerations When Operationalizing Revenue Management
Pharmaceutical manufacturers commonly invest a considerable amount of time, money and additional resources into revenue management. Most would likely say they desire to invest even more because of the significant impact it has on their products’ gross-to-net. But how and where to invest valuable assets is a significant decision. The process of revenue management, including […]

Digital Guide to Commercializing Complex Therapeutics
Pharmaceutical manufacturers specializing in Rare Disease, Oncology, Personalized Cancer Immunotherapy, and Cell and Gene therapies encounter a range of complex challenges, including advancements in medicine and technology, changing patient and provider needs, market access, regulatory pathways, pricing transparency, patient reach, patient adherence, and ever evolving disease states. Despite spending over $200 million on product launches, […]

Past and Present U.S. Public Health Laws and Regulations, and Their Impact on the Corresponding FDA Regulated Products and Industries
To be regulated by the FDA, foods, cosmetics, human and animal drugs, biologics, tissues, medical devices, combination products, and tobacco products have to meet the federal public health definitions. This white paper provides an overview of past and present U.S. public health laws and regulations and their impact on FDA-regulated products and industries. It highlights the […]

Pricing Complexities of a Combination Therapy
This article, authored by EVERSANA’s Asia Pacific team, discusses the increasing use of combination therapies in oncology and the challenges associated with pricing them. Combination therapies, which involve a backbone therapy and one or more add-on therapies, offer better clinical outcomes but pose difficulties in determining their value and pricing. One challenge arises from the fact […]

Integrating the Pillars of Global Pricing Governance
How Emerging Trends are Demanding a More Holistic View to Drive Maximum Value As the complexity of investments needed to secure patient access to therapy increases, so does the impact of these investments on a company’s net revenue. Each decision needs scrutiny, not only on its own merits and how it can affect patient access […]

Seven Challenges Traditional Omnichannel Tactics Cannot Overcome
How pharma companies utilize omnichannel is a significant factor in their brand’s success, especially as traditional efforts continue becoming more and more obsolete and rejected by patients and providers. Many manufacturers are facing pivotal decisions about what direction to go in with their omnichannel efforts, and whether to embrace new innovative techniques. In this article, […]

The Future of Omnichannel is Here. Don’t Get Left Behind.
The standard for omnichannel continues to evolve whether pharma companies like it or not. A next-gen approach to this crucial component of the patient journey is essential to maximize success and best optimize valuable assets. In this article, learn how redefining omnichannel efforts does not have to require investing additional resources. Instead, companies can adapt […]

Create a Comprehensive Experience by Optimizing Forward-thinking Omnichannel Strategies
Deploying successful omnichannel strategies requires manufacturers to evaluate their tactics and ensure they are meeting patients’ and providers’ needs in a modern and effective way. In this article, we lay out a check list of criteria for manufacturers to incorporate into their omnichannel strategy for heightened results that will elevate their brand among competitors, best […]

Role of Decentralized Trials for Newer Drug Approvals
Decentralized clinical trials (DCTs) are an innovative approach to conducting clinical research where aspects of the trial are carried out remotely, allowing patients to participate from their homes or local healthcare facilities. DCTs offer several advantages over traditional trials, including increased patient participation and diversity, improved convenience and flexibility for patients, and faster, more efficient […]
Navigating an Ever-evolving Canadian Market Access and Reimbursement Environment
These are just some of the trends currently shaping the Canadian market access environment. Achieving favourable market access and reimbursement outcomes for new treatments in this ever-changing landscape requires intimate knowledge of the Canadian landscape, processes and key stakeholders, careful strategic planning, sound tactics and skilled execution. Some key steps and considerations to maximize the […]

2023 Outlook Of China Healthcare Industry: Remaining Pragmatic While Moving Up The Value Chain
As we enter 2023, China surprised the world with a swift and decisive shift in COVID policy, demonstrating the country’s ability to be flexible and pragmatic in its decision-making. In contrast to Russia’s persistent adherence to ideologies, China demonstrated its willingness to pivot quickly when necessary. The 14th Five-Year Plan at the 20th National Congress […]
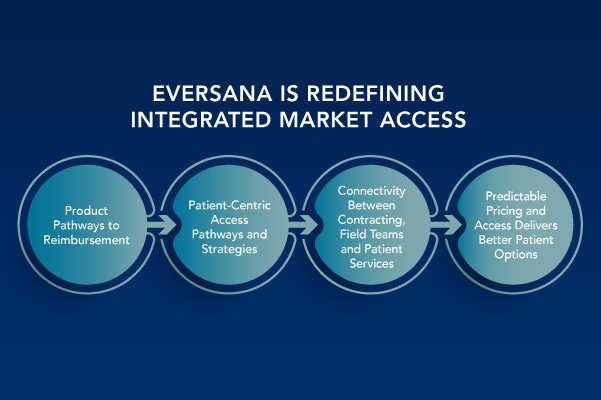
Employ an Integrated Market Access Strategy to Deliver Brand Success
The pharma/life sciences pipeline is vibrant, with ongoing innovation bringing new hope to patients and providers across many therapeutic areas. Against the backdrop of rapid scientific advancement in and precision medicine and targeted therapies in primary and specialty care as well as rare and orphan diseases, today’s medicines continue to create immense complexity for all […]

What EVERSANA’s Growing Abstract Count at ISPOR U.S. Means to the HEOR Industry
EVERSANA’s Value & Evidence (V&E) and Data & Analytics (D&A) team collectively had an impressive 27 abstracts accepted to ISPOR U.S. 2023, showcasing EVERSANA’s ability to cross-solve for clients’ HEOR needs. EVERSANA’s Value & Evidence and Data & Analytics teams have had 27 abstracts accepted to ISPOR U.S. 2023, the leading global conference for health […]

Use of Machine Learning to Accurately Size Market Potential and Optimize Sales and Marketing Resources
Machine Learning as a strategic lever in healthcare. Recent advances in Machine Learning (ML) now make it possible for pharmaceutical companies to more accurately identify patient populations, including misdiagnosed patients with rare diseases and other diseases that have a high prevalence of symptomatic related diagnoses, along with their healthcare providers. With this knowledge, it also […]

Case Study: Optimizing use of Field Reimbursement Managers
Navigating the complexities of insurance coverage can be a time consuming and challenging task. Field Reimbursement Managers (FRMs) were introduced as a way to help healthcare providers address issues or challenges related to securing timely access to treatments for patients, and for securing timely and appropriate reimbursement for therapies that the practice must purchase and […]

Case Study: Waste Reduction through Packaging Optimization
Pharmaceutical companies are being challenged to develop more effective delivery mechanisms of their therapies in order to reduce the amount of product waste. Recent changes in legislation, including the Waste Reduction Act of 2022 and adoption of Section 1847A of the Social Security Act have made it critical for pharmaceutical companies to optimize drug packaging […]

Adult Vaccine Landscape in APAC: The Need for Change
Content for this article was contributed by EVERSANA’s Asia Pacific team. Do Adults Need Vaccines? Immunization should not end with adulthood. Using immunization to prevent infection should be a life-long process, as adult vaccination provides benefits at both individual and country levels, reducing the overall burden of preventable diseases. However, in practice, adult vaccinations are […]

Access Challenges for Severe Infectious Diseases
Content for this article was provided by EVERSANA’s Asia Pacific team. The global burden of infectious diseases is increasing, especially for severe diseases such as invasive aspergillosis, a rare infectious disease, which has a 90% mortality rate. These diseases have poor prognoses and high mortality rates. Disease severity and mortality rates have been increased by […]

Emerging Trends in the APAC MedTech Market
Content for this article was contributed by EVERSANA’s Asia Pacific team. APAC’s MedTech market will soon be the second largest in the world. This growth presents both opportunities and challenges across the region. Booming APAC MedTech Market: The best time to enter is now Over 1.3 billion people will enter their sixties by 2050 in […]

Challenges (and Solutions) to Rapid Omnichannel Transformation
Omnichannel can offer countless benefits and notably improve provider and patient journeys. But current marketplace challenges are disrupting success with omnichannel efforts for some manufacturers, and hindering their quality of service to healthcare professionals and the patients they serve. In this panel discussion from the 2022 Digital Health Coalition and moderated by Aaron Uydess, EVERSANA […]

Evolution of Digital Apps to Total Wellness Solutions
Content for this article was contributed by EVERSANA’s Asia Pacific consulting practice. There is a remorseless demographic logic to increasing health costs. Life expectancy increases, the population ages, and “lifestyle” diseases penetrate further and earlier. How can public health stakeholders resist the trend? One intervention point is promoting the benefits of healthy, active lifestyles. Government-backed […]

Benchmarking Your Specialty Brand’s Rx Pull-Through Against Competitors In and Out of Your Network
As an executive at a specialty drug manufacturer, you know having access to your brand’s pull-through key performance indicators (KPIs) inside your network is only a very small part of a much bigger picture. In order to know what good really looks like, you need to know two things: How your brand is performing when […]

The Evolution of HTA and Its Impact on Drug Prices in Japan
Health Technology Assessment Status in Japan Health technology assessments (HTAs) are increasingly used worldwide to assess the clinical and economic impact of drug treatments and technologies, inform health policy, and guide drug pricing and reimbursement. In Japan, HTA occurs after the debut of a product and when companies enter the system, whereas in the UK […]

How Specialty Pharmacies Can Elevate Your Channel and Distribution Strategy for HCP-administered Buy & Bill Products
A successful channel and distribution strategy for Buy & Bill (B&B) products should be centered around the partners you sell to and through. That means considering any third-party logistics (3PL) providers, specialty distributors (SD) and specialty pharmacies (SP) who will touch your product between the manufacturing/packaging site and patient administration. Taking a holistic view of […]

Plasma derived medicinal products – What is the way forward?
Content for this article was contributed by the EVERSANA Asia Pacific team. Plasma-derived products have a long history of providing benefits, especially in fractions addressing coagulation. Plasma is a rich biological substance and one of the main components of blood, constituting about 55% of total blood volume. The worldwide plasma protein market is expected to […]

Single-Cell Multiomics: Opportunities, Challenges, and the Evolving Business Model
Content for this article was contributed by EVERSANA’s Asia Pacific team. Single Cell Multiomics is a Fast-Growing Market Space with a Wide Range of Applications Single cell multiomics is a new and exciting space in the life sciences industry and research community. Advances in single-cell isolation and barcoding technologies have provided opportunities to profile DNA, […]

Successful Early-Stage Out-Licensing Requirements
The attention on vaccines during the COVID-19 crisis highlighted the role of biotech companies and positive sentiment increased for the sector. In 2021, a record number of biotech (100+) companies went public and listed companies soared to an all-time high. However, today investors have reset their expectations for the sector considering the current economic trends. […]
EVERSANA’s Value & Evidence Team’s Work Tops List of Most Read Articles in 2021 in Journal of Comparative Effectiveness Research
If you work in the field of Health Economics and Outcome Research (HEOR) in the life sciences industry, you know just how valuable the Journal of Comparative Effectiveness Research (JCER) is. The publication features peer-reviewed studies from HEOR teams across the globe and is the go-to for researchers and scientists to stay on top of […]

Case Study: Leveraging Machine Learning Models in Patient Identification and Behavior Prediction for Rare Disease Indications
When a mid-sized pharmaceutical company was challenged with trial and adoption of their immune thrombocytopenia (ITP) drug due to fierce competition and the lingering impact of COVID-19, they turned to the team of data and analytics experts at EVERSANA to help them overcome this challenge. In this latest case study, see how the use of […]
Reimagining the Patient Care Ecosystem
Improving Access, Removing Barriers and Speeding Up Time to Therapy Patient demand for telehealth and a flood of industry challenges are disrupting the traditional healthcare landscape. Lack of patient awareness, access to care and overburdened clinicians have created significant wait times to see a provider and a prolonged journey to diagnosis and treatment. The lengthy […]
Customer Centric Approach to Medical Affairs Leveraging Digital Technology
As technology advances the needs of healthcare professionals (HCPs) and patients evolve. The onset of the COVID-19 pandemic accelerated digital adoption among these groups. Medical Affairs departments now require a digital strategy in addition to medical strategies, including technology and innovation roadmaps to offer omnichannel solutions to ensure communications are occurring via the customer’s preferred […]
EVERSANA’s Continued Commitment to Rare Disease
World Orphan Drug Congress USA 2022 As a continued commitment to the rare disease space, EVERSANA was a proud sponsor and participant of this year’s World Orphan Drug Congress USA (WODC). Leaders including Maria Kirsch, General Manager, Patient Services, Simon Andrews, Senior Vice President, Head of Analytics, and Scott Snyder, Chief Digital Officer attended and […]

Is Telemedicine Here to Stay?
Prior to the COVID-19 pandemic, healthcare delivery had been predominantly face-to-face. COVID-19 exposed the capacity limits of the healthcare system, and forced the system to optimize its methods of healthcare delivery by considering non-conventional methods such as telemedicine. Download our article to learn how our telemedicine matrix can inform companies’ mid-to-long term brand strategies.

Established Pharma Brands – A Good Investment?
Offering high cash flows with low clinical and commercial risks, established brands are gaining attention. Big pharma, such as Merck, GlaxoSmithKline (GSK), Pfizer and Sanofi, are spinning off their established brand drugs into new companies. Merck announced that it would be creating a new company, to house its women’s health, well-known established brands and biosimilar […]

An Innovative Approach to Comprehensive Key Opinion Leader (KOL) Mapping
Traditional key opinion leader (KOL) mapping techniques are heuristic and often haphazard: EVERSANA™ has developed an innovative and robust approach that dramatically improves results. We base our ability to identify and prioritize KOLs across disease areas on new technologies and datasets that have made it possible for us to customize searches for many client objectives, […]

Evolution of Integrated Mental Health Treatment Models in Southeast Asia (SEA)
Implement Mental Health Integrated Care Models with A Trusted Partner Integrated-care models for mental health conditions implemented in China and Japan have demonstrated the efficacy and cost-effectiveness of such models. SEA is the next frontier of implementing these models and stakeholders in SEA countries have begun assessing and implementing such models. However, infrastructure, numbers of […]

Challenges of Scaling Up Cell Therapy to New Geographies
Cell therapy has emerged as one of the most promising disruptive innovations in the pharmaceutical industry. However, despite its commercial promise, companies developing cell therapies have not been able to take full advantage of its massive potential. Cell therapies offer a massive commercial opportunity In 2021, the cell and gene therapy (CGT) market was valued […]

Booming but Constrained: Digital Therapeutics for Mental Health in APAC
Digital therapeutics (DTx) are a fast-emerging class of therapies that use software to treat disease, both as standalone treatments and for treatment optimization. Applications range from improving patient adherence to supporting physicians in managing patients remotely. DTx has been much hyped as transformational across the healthcare ecosystem by bringing personalized medicine to all through AI […]

Need for RWE in Consumer Health and Its Impact in Managing Lifecycle of Consumer Health Products
Healthcare has never been more important to the world than in the past two years and it will remain central during the economic recovery. The pandemic’s impact on medical and healthcare systems has emphasized the value of real-world evidence (RWE). This value is now becoming apparent in consumer health, as well as its traditional applications […]

Is Your Digital Communication Strategy Up to Par?
In today’s competitive global market, pharmaceutical companies cannot afford to waste time or resources on strategies that do not fit patient needs or generate scripts. Interconnectivity between all stakeholders and a 360° view of everything that touches the patient are essential when mapping each patient’s and HCP’s unique and differentiated journey. But how do you […]

Case Study: Defining the Rare Disease Patient Journey to Support Commercialization of Therapy
Rare diseases are complex, and with limited research available, therapeutic options are often limited. Gene therapy, a growing area of clinical research, is showing great promise in treatment that may be life-altering for patients with many rare diseases. A client specializing in gene therapy for a particular rare disease came to EVERSANA looking to better […]

Demystifying Data and Analytics – How to Advance Clinical and Business Objectives
The need to develop a strong, comprehensive data-analytics strategy to support pharma and healthcare initiatives cannot be stated strongly enough. Data is unbiased; it can validate hypotheses or provide direction. But the primary goal is to use data analytics and modeling to develop a more accurate view of what’s really going on in the lives […]

FDA Moves Cybersecurity Into the Product Life Cycle
Due to rising cyber-attacks and the potential to cause harm to patients, medical facilities and hospitals, the U.S. Food and Drug Administration (FDA) has recently increased scrutiny of cyber controls in FDA premarket submissions of medical devices. Manufacturers must prove that devices, including software-as-a-medical device (SaMD), do not present cybersecurity vulnerabilities that may affect the […]

Impact of Patient Reported Outcome Measures on HTA Decisions for Rare Diseases
As rare diseases become an increasing area of global focus, pharmaceutical manufacturers are taking a closer look at how patient-reported outcomes (PROs) may be used to improve uptake in HTA evaluations. Although “rare” suggests not many people are affected with a condition, in the EU between 6,000 and 8,000 different rare diseases affect an estimated […]

EVERSANA Featured in NIH Study Detailing the Cost of Treating Rare Disease
“The IDeaS initiative: pilot study to assess the impact of rare diseases on patients and healthcare systems” EVERSANA™ was the only data and analytic organization invited by a consortium of provider organizations and the NIH to participate in a study with the National Institutes of Health’s National Center for Advancing Translational Sciences (NCATS). The study, […]

What You Need to Know as the European Union Embarks on Joint Health Technology Assessment (HTA)
Facing the “Fourth Hurdle” Member states of the European Union benefit from a centralised marketing authorisation process for medicinal products. Also, since its implementation in 1993, the European Medicines Agency (EMA) has assured pharmaceutical companies the right to commercialise products that underwent a centralised marketing authorisation in the second largest (and most diverse) single pharma […]

How Do We Pay for a Cure? How to Put a Price on Life-changing Treatments
The global cell and gene therapy market is expected to reach $13.8 billion by 2026, expanding at a compound annual growth rate of 12.4%. — Global Cell & Gene Therapy Manufacturing Services Market by Type – Forecast to 2026 Report With over 2,000 ongoing clinical trials in regenerative medicine, patients across the world will soon […]
Gene Therapy in Today’s Digital World
Rare diseases are an emerging global public health priority, with a staggering impact on the more than 300 million people worldwide who are living with them. There are more than 6,000 rare diseases, 72% of which are genetic, and 70% of those genetic rare diseases start in childhood. Gene therapy holds promise for treatment, and […]

How Can Utilizing Digital Therapeutics Provide Better Care to the Veteran Population?
EVERSANA’s Joe Perekupka, Chief Commercial Officer, participated in a keynote panel discussion at the recent DTx West Summit: “How Can Utilizing Digital Therapeutics Provide Better Care to the Veteran Population?” Joe was joined by moderator Debra Reisenthell, Founding CEO, Freespira; and panelists Ryan Sadlo, VP, Growth, Wellsheet; and Ruth Lowenthal, VP, Total Rewards, Xcel Energy. […]

Understanding Current Trends and Needs: Medical Information Contact Center Services
“Understanding Current Trends and Needs: Medical Information Contact Center Services,” a poster by EVERSANA’s Michael DeLuca, Senior Vice President, Medical Affairs, and Keyur Brahmbhatt, Director, Medical Affairs, along with Evelyn Hermes-DeSantis, Director, Research and Publications, phactMI, explores the significant role Medical Information (MI) plays in addressing inquiries from healthcare professionals, payers and patients and ensuring […]

Succeed When Others Fail: Avoiding 20% in Investment Waste During Launch
It’s a long-standing perception that pharma overspends during launch, but we did not have the data or methodology to understand or validate this notion. Until now. New Research by the Numbers 10: Recent analysis across 10 traditional launches where the manufacturer opted to commercially launch a new drug $345M: The total cost for a manufacturer […]

Importance of Impactful Medical Information Content
Medical Information (MI) services have evolved as technology has advanced and healthcare professionals’ and patients’ needs and preferences have changed. Understanding the audience, identifying the most beneficial content format, and utilizing the preferred channels of communication are crucial for an impactful medical strategy. Pharmaceutical companies must have technology and innovation roadmaps for omnichannel solutions to […]

How to Ensure Speed and Efficiency When Launching in Europe
Today is an extraordinary time for medical innovation. The pharmaceutical industry is yielding protection against diseases, focusing more on rare and complex disease treatments, and the world is getting better health outcomes. Global life expectancy is more than 70% higher today than it was in 1960 as diseases that were once considered terminal are now […]

A Holistic Launch in Europe Is Possible, But Change Is Necessary
Unless you live in Europe or have commercialized a product in this region, you may not realize that the European pharmaceutical market is a huge contributor to global health, and it is currently undergoing market-wide changes. Here are a few key facts to know about Europe’s pharmaceutical markets: Europe’s population is more than double that […]

How to Eliminate Access, Affordability and Adherence Barriers in 2022
Today, everyone expects both digital and human outreach, and manufacturers face the challenge of trying to strike the right balance between tech and in-person touchpoints to enhance patient and healthcare provider (HCP) engagement. Despite all the clear benefits of digital interactions, 40% of customers prefer speaking to a real person on the phone; and for […]

QUIZ: How Mature Are Your HCP & Patient Engagement Strategies?
In the increasingly virtual world we live in, there’s no denying the instrumental role that technology plays in engaging patients and healthcare providers (HCPs). With heavier reliance across industries on text alerts, automated phone reminders and digital ads that bring product promotion directly to consumers, people expect this same level of digital outreach for their […]

A YEAR OF ACTION: Why Data-Backed, Integrated Commercialization Strategies Are Must-haves in 2022
By applying transformative commercialization models, nurturing digital transformation and trailblazing in global expansion, we can get therapies to patients around the world who are still waiting for treatment options. For the past two years, the pharmaceutical industry has proven that it can adapt to change. In 2020, pharma pivoted to manage the coronavirus pandemic, and […]

Client Story: An Ode to EVERSANA
At EVERSANA, putting our clients and patients first is at the forefront of everything we do. Recently, when EVERSANA ENGAGE’s Bausch & Lomb (B&L) team closed out their last event of their speaker series, they didn’t just receive any feedback. They received a poem. Jodi Ceberio, Managing Director, Client Services, said that this poem is a […]

Pharma Europe 2021 : Panel
A Data and Digital Enabled GTM Strategy for Long-term Business Impact Rohit Sood, Executive Vice President, COMPLETE Commercialization, joined a panel of industry experts to discuss why an aligned and cross-functional go-to-market strategy has never been more important and how to balance strategic priorities in this Pharma 2021 conversation. Watch the recording: Meet with our experts.

Bio-Europe “Collaboration Close-Up”: Exploring the EVERSANA – Shorla Pharma Partnership
Launching in today’s unpredictable market is a journey with new challenges around every corner. While the oncology pipeline is rapidly growing, stringent competition and an evolving provider environment are putting intense pressure on manufacturers to build effective commercialization infrastructures and launch products at unprecedented speeds, which comes at a steep price. In this session, Shorla […]

The Need for RWE Studies in APAC and the Impact on Product Life Cycle Management
Advances in technology and globalization have enabled significant growth in the pharmaceutical industry over the last decade. Digital initiatives and focusing on patients have also conditioned researchers to think beyond traditional data collection to support product value propositions. As a result, real-world evidence (RWE) is gaining in importance and becoming an important part of managing […]

Webinar: Coupon & Co-Pay Conference 2021
Leveraging eServices and Technology to Improve Access and Co-pay Card Utilization Payers and self-insured employers are driving enrollment in high-deductible health plans, shifting financial risk to patients and increasing out-of-pocket costs. As a guest presenter at Informa’s annual Coupon and Co-Pay 2021, EVERSANA’s Tom Doyle outlined how manufacturers can leverage predictive analytics, omnichannel and personalized engagements to ensure […]

Health Technology Assessment in the Asia Pacific Region
Health technology assessment (HTA) is the systematic evaluation of the properties and effects of a health technology, addressing the direct and intended effects of the technology as well as its indirect and unintended consequences. In many countries, it is a primary evaluation tool for making pricing and access decisions, providing a platform for understanding economic […]

EPP Studio Live Webinar — Impact of COVID-19 Pandemic on German Healthcare System
Leading up to the recent German elections, the topic of healthcare took center stage for the aging German population. With nearly 90% of the population participating in Statutory Health Insurance (SHI), increased financial pressures have widened the gap between SHI coverage and anticipated expenditures. During this EPP Studio Live Webinar, Douglas Foerster, Senior Vice President […]

Webinar: Discover Patient Support Program Needs for Patients with Rare Diseases
In a world where patients face more medication barriers than ever, patient support programs for rare disease patients are critical in providing value-based care that yields a palpable and lasting impact. Manufacturers are acutely aware that product access and affordability is key to improving patient adherence and are more than willing to allocate the time […]

EPP Studio Live Webinar — EU Joint Procurement of COVID-19 Vaccines: A Model for Future Market Access in Europe?
The COVID-19 pandemic has changed the way we live and the way we respond to global healthcare crises. During this EPP Studio Live webinar, Douglas Foerster, Senior Vice President of Pricing & Market Access for EVERSANA, shares a brief historical summary of how the European Union negotiated the acquisition of COVID-19 vaccines through an unprecedented, […]

A Data-Driven Commercialization Pathway to Expedite Rare Disease Diagnosis and Adherence
“We often think about big data as this big, cold, robotic thing; and I think it reveals, in real time to us daily, that there are opportunities for many small, important moments and many impactful, emotional things to happen for patients and caregivers.” —Amy Hutnik, General Manager, Agency, Advisory & Evidence Services When the life […]

Building a Life Sciences Digital Ecosystem
In the life sciences world, we hear more and more of the requirement for digital ecosystems in various therapies. What do we mean by these, and what benefits do they provide? A digital ecosystem is a network of multiple stakeholders who collaborate to enhance delivery to patients. A healthy, sustainable ecosystem benefits patients, prepares companies […]

Assessing Market Opportunity and Entry Challenges in Mental Health Disorders
Although mental health disorders affect almost 264 million people worldwide, they are often misunderstood and misdiagnosed, and access is poor. The World Health Organization (WHO) reports that 80% of people in low- and middle-income countries receive no treatment for their mental health disorders. As well as being underdiagnosed, mental health disorders are also poorly studied […]

Increasing the Value of Community Health Workers
In developing countries, frontline community health workers (CHWs) extend basic healthcare services to communities. These extensive CHW networks provide services that improve various health indicators. The Philippines has set a goal of one CHW (“Barangay Health Worker”) in every village, while India is close to meeting its goal of one CHW (“ASHA”) per 1,000 people […]

Getting Treatments to Patients When They Need Them Most: Next Gen Commercialization in Oncology
Commercializing oncology products is extremely complex, but successfully launching new therapies to market is essential for patients and providers in the fight against cancer. In 2020, about 10 million people died from cancer, proving even in a global pandemic that cancer continues to be a leading cause of death globally. While the oncology pipeline is […]

Novel Antibiotic Opportunity in China
China, with its large population and extensive healthcare system, is the second-highest user of antibiotics in the world. Such widespread use has inevitably led to high levels of antibiotic resistance in the population. When isolated, it becomes clear that a significant majority of the infecting bacteria are gram-negative. Within these bacteria, antibiotic resistance has grown […]

Assessing the Feasibility of ITCs
When looking at different healthcare interventions for a disease, there may be limited direct evidence on how the treatments compare with each other in terms of their efficacy, tolerability or another measure of interest. In this video, EVERSANA’s Chris Drudge, MPH, PhD, Associate Project Manager – Value and Evidence, shares how indirect treatment comparisons (ITCs) […]
Simplifying EU Distribution to Maximize Cost Efficiency and Speed to Market for Patients and Manufacturers
COVID-19 ignited a spark of innovation in the healthcare industry, forcing global markets to reconsider drug development and commercialization processes. The European Union (EU), specifically, is taking carefully planned steps into a new phase of pharma with recent changes, including the Pharmaceutical Strategy for Europe. But one element of the European pharma industry that remains […]

Digital Therapeutics: Where Technology Meets Healthcare
Digital health is growing rapidly across the world, supported by technologies that not only assist disease monitoring or prevention but can also reduce the healthcare burden. Digital technologies have improved equity of access and enabled European patients to monitor and self-manage their health. It has improved efficiency, effectiveness and quality of patient care while also […]

How Manufacturers Can Optimize and Organize Support Services for Specialty Drugs Through a Patient Touchpoint Analysis
Currently representing almost half of the overall pharmacy benefit spend, specialty drugs are classified as being high-complexity, high-touch and high-cost medications. As healthcare providers (HCPs) and patients turn to these more expensive products to treat rare, complex and/or chronic medical conditions, the need for increased patient education, adherence and support services has been elevated. To […]

Adapting Patient Access Programs to Asia
In Western markets, pharmaceutical companies have developed many approaches to patient access programs (PAPs), normally emphasizing initiatives that, while helping patients, also support pricing and reimbursement initiatives, such as: Early access programs before market authorization. Financial access initiatives before achieving reimbursement. Value-based contracts, managed-entry agreements or outcomes-based programs for achieving reimbursement. Non-financial assistance post-reimbursement. The […]

Optimizing Your Digital Marketing Spend and Field Deployment to Impact Script Adoption
In today’s competitive global market, pharmaceutical companies can’t afford to waste time or resources on strategies that don’t generate scripts or fit product and patient needs. Without data-informed, fully integrated campaigns, there’s a missed opportunity to mine deep insights that can inform next steps and accurately pinpoint efforts to doctors and patients who could benefit […]

Competitive Benchmarking In Trade – Answering The Who, What, Why and When
Last month our colleague Derek Cothran addressed the importance of using secondary research to benchmark your Patient Support Program (PSP) against obvious and not-so-obvious competitors. We’d like to continue a discussion around competitive benchmarking in this month’s blog, but this time focused on how it can be used to optimize a channel or distribution strategy […]

Navigating the Transition to Post-Approval Pharmacovigilance With EVERSANA and ArisGlobal
Partnering with a contract research organization (CRO) provider during clinical development fills an important role in the product life cycle, providing pharmaceutical companies with pharmacovigilance expertise and support during clinical trials. After product approvals, it can be tempting for companies to remain with their CRO partner; however, the commercial phase of the product life cycle […]

Case Study: Launching a New Therapy for HER2-Positive Metastatic Breast Cancer
When MacroGenics partnered with EVERSANA, they had less than five months to launch their first product in the midst of the global pandemic. To meet their timeline and streamlined launch, MacroGenics needed a commercialization partner with an end-to-end platform that would allow them to build their capabilities expeditiously and strategically.

The Patient Access Paradox: How the New CMS Rule Could Prioritize Drug Pricing Before Clinical Decision-Making
In January 2023, co-pay programs will be put to the test, consequently examining how well your brand can adapt to the Final Rule changes to meet patient and provider needs. Our recommendation: Don’t wait – start solutioning a patient-centric approach to access and affordability now.

A SpaceX Philosophy to Launching in Pharma
In thinking about the economics around the launch of pharmaceutical products, it is useful to compare the situation to another area that has seen its economics evolve in recent years: space travel. For decades, the only reusable space vehicle was NASA’s space shuttle. When the space shuttle was in operation, it could launch a payload […]

Considering a Virtual Advisory Board as a Viable, Cost-Effective Option for PMR Planning
Advisory boards are key in helping pharmaceutical manufacturers refine product positioning and guide value messaging throughout the product life cycle. Like many processes this year, advisory boards have been impacted by the pandemic’s travel restrictions and have been unable to convene as traditional in-person meetings, but the need for their thought leadership remains. As manufacturers adapt, leaders might feel limited to multiple one-on-one discussions with board members or be struggling with the number of challenges associated with virtual meetings: […]

The Rise of Digital Therapeutics Opportunities in China
The expansion of digital health technologies is growing exponentially and has increased through the pandemic. One focus for investment for this therapeutic area is China, where digital growth has outpaced most of the western world in areas such as e-commerce. While such growth is exciting, business models that offer returns are less understood. EVERSANA’s global commercialization experts explain how we’re examining the Chinese market to help our […]

Transform Your Medical Information Contact Center Into a Strategic Asset for Customer Engagement
The pharmaceutical industry is constantly evolving, and manufacturers are re‑strategizing, embracing technology and innovation, to meet the needs of the providers and patients they serve. Successful product launches on a global scale are critical for manufacturers to reach more patients and providers. As a result, manufacturers are streamlining operations, relying on fewer vendors and service […]

PharmaVOICE Webinar: Next Gen Commercialization Model for Oncology
In a new 60-minute virtual panel, “Next Gen Commercial Models in Oncology,” PharmaVOICE Editor Taren Grom sat down with industry leaders to discuss how changing market dynamics and a rich pipeline in oncology are creating a need for next gen commercial models.
Ask the Expert: One-on-One with Amy Hutnik
The pharma industry’s needs have evolved beyond one-dimensional playbooks, disconnected promotional efforts and limited ability to assess stakeholder engagement. Instead, manufacturers need to adopt a comprehensive omnichannel model that allows for data-driven planning and real-time analysis of results from marketing campaigns, field activities and patient services programs to create a cohesive brand experience with maximum […]

Pharma USA Webinars: Personalize HCP Experiences for Deeper Engagement
EVERSANA was proud to participate in two Pharma USA 2021 sessions. This webinar, featuring EVERSANA’s Amy Hutnik, General Manager, Agency, Advisory and Evidence Services, covers the following topics: Harnessing AI to inform your sales approach and meet your customers’ need for both human interaction and digital engagement. Pull don’t push: Build more meaningful relationships with […]
Engaging Oncology Patients and Providers in The Age of COVID And Beyond
The engagement model for oncology patients and providers is failing, and challenges brought on by the COVID-19 pandemic have only accelerated the trend. Not only are pharmaceutical reps restricted in the types of information they are allowed to communicate, but their access to providers in oncology practices is also more limited than ever as a result of COVID and productivity concerns.

Are You Benchmarking Your PSP Against Others? If So, Who and What Are You Comparing It Against?
Many of our clients are looking for ways to ensure their Patient Support Program (PSP) is not only serving their patient and provider populations well but that it is doing so in a fiscally responsible way. Additionally, they are seeking validation that their PSP is at least at parity or superior to the competitor products’ […]
Informing NAMs Payer Strategies and Improving Contracts With Integrated Commercial Services
Pharmaceutical manufacturers rely on national account managers (NAMs) to build relationships, negotiate contracts and get their products in front of payers and pharmacy benefit managers who will clear the way for patient treatment coverage. Challenges Facing NAMs Typically, NAMs rely on the customer or the health plan to direct contracting strategies and product expectations, and […]
Revolutionizing FRMs With Data Connectivity and Commercial Services Integration
FRMs, sometimes referred to as field reimbursement specialists or patient experience specialists, are the key drivers in patient pre-authorization and billing and coding processes, and play a vital role in the prescription and adoption of specialty drugs. While field reps focus on selling, FRMs focus solely on supporting healthcare provider (HCP) offices and helping office […]

3 Ways Field Teams Can Shape the Market Before and After Product Launch
“It is not how much you have or know, or even who you know; it is how well you adapt to the inevitable changes along the way. As Heraclitus, the Greek philosopher, said, ‘Change is the only constant in life.’” – Marc P. Bernarducci, Senior Vice President of Field Solutions – Clinical Field Medical (FM) […]

The Secret’s Out: The Impact of Net Price Transparency
Pricing system pressures are increasing for the top four markets in Europe. Price transparency, changes to public health plans and curative therapies are set to disrupt traditional drug pricing. To navigate this ever-shifting landscape, companies must carefully consider product adoption and launch sequencing, and understand the potential impact that net price transparency might have. In […]

Audit From Anywhere: Upgrade Your Quality System Audit Programs
As the world strives every day to adapt to changes this year, one change that pharma manufacturers are adapting to is the impact of COVID-19 on product safety audits. From supplier audits to evaluating internal or affiliated sites with the FDA, travel restrictions have made it difficult to access and be on site for audits at facilities as has been […]

Are You Tracking the Performance and Satisfaction Ratings of Your Patient Services?
The types of services patients need for support change and fluctuate over time, particularly as a product moves through its lifecycle. The patient services necessary to drive access, affordability and adherence to your product in the launch phase can be markedly different from the patient services that accomplish the same goals at later stages of […]

Visualizing EVERSANA’s Omnichannel Activation Model
The pharma industry’s needs have evolved beyond one-dimensional playbooks, disconnected promotional efforts and limited ability to assess stakeholder engagement. Manufacturers need to adopt an omnichannel model that goes beyond “marketing” to provide actionable insights that better inform commercial strategies and elevate brand success. Expect more from your investments in datasets, technology and promotional campaigns; expect […]

Webinar: Under Pressure: Global Trends in Net Price
In the past few years, net price transparency has become a subject of debate in the international pharma community. As pricing reforms and shifting pressures on pricing systems continue to intensify, how will they impact markets in Europe and Asia-Pacific? Watch our webinar featuring EVERSANA’s Alan Crowther and Amardeep Udeshi for a discussion (presented at World EPA Congress […]

Turning Theory Into Action: How Real-World Evidence Drives Clinical Research and Improves Patient Outcomes
Life sciences companies have made significant investments in real-world data (RWD), but most established providers can deliver only a fraction of what’s needed to be impactful. Breaking down traditional healthcare silos for a more innovative approach to drug development can shorten the research and development timeline while substantially reducing wasted investments along the way. From […]

How to Scale Your Learning & Development Department: Ask The Expert
For years, Learning and Development teams have been asked to “do more with less.” When budgets tightened, teams would get more creative allocating their resources and time. But today – in the midst of changing market dynamics and a global pandemic – teams have been asked to “do more for more.” They are finding themselves […]

Webinar: CMS Final Rule on Cost-Sharing Assistance
The CMS final rule on drug reimbursement addresses how co-pay coupons and vouchers are being exempted from deductibles by PBMs. The rule seems to ensure that the full value of manufacturers’ coupon and voucher programs accrue to the enrolled patient, meaning that the portion of the cost of the drug paid by the manufacturer program […]

Change Is Happening in the EU: What Pharma Companies Need to Know About the European Union’s New Pharmaceutical Strategy
For the first time, the European Union is undergoing a major overhaul of its pharmaceutical industry with the new Pharmaceutical Strategy for Europe, which was launched in late 2020. Through this strategy, the European Union (EU) is making changes to its infrastructure with the goal of building a holistic, patient-centered, forward-looking pharmaceutical landscape for all EU member […]

Watch Now: PharmaVOICE Panel on Next Gen Patient Services Models
The Future of Data-Driven Patient Support Is Now The use of health data technologies and analytics in the life sciences industry continues to evolve, but many manufacturers are still crawling around in the dark. With limited in-person patient and provider-rep interactions, hubs that lack synergized technology and data are struggling to understand access and adherence […]

Leveraging RWE to Enhance Experiences Across the Patient Journey
In a tech-driven healthcare ecosystem in which patients are engaged consumers, product value is contingent on treatment outcomes and patient success. With digital tools and resources at their fingertips, today’s patient is empowered to make informed treatment decisions; and physicians need enhanced support to ensure they’re meeting patient needs. Providing physicians with the tools to […]
Reinventing Launch: The Gold Standard Of Drug Commercialization
In a world that is rapidly changing, we must evolve beyond traditional strategies to create true impact for patients. EVERSANA’s complete end-to-end commercialization model enables manufacturers to bring their drug to market at a fraction of the cost of “going it alone” or partnering with another pharmaceutical company.

The Crush: How Covid-19 Is Impacting Mature Brand Revenue & Long-Term Value
The impact of COVID is reverberating across all aspects of society and business. In the healthcare industry, hundreds of thousands of patients are not getting proper access to and utilization of therapies that can improve their clinical outcomes. The clinical consequences of this will lead to compromised patient outcomes and further healthcare cost increases. This […]

How to Determine Price and Global Launch Sequence in a Post-COVID World
The globe is facing a multitude of governing and legislative changes that will directly affect pharma and pricing in coming years, with many of these changes initiated by the pandemic. As countries become increasingly interconnected, decisions in one pharmaceutical market will have ripple effects globally.

Transforming Pharma With Industry-Leading Partnerships
EVERSANA is filling an influx of essential commercialization needs to overcome strained resources and support COVID-19 treatment launches. From groundbreaking treatments to long-overdue therapy improvements, there will be at least five new treatments headed to market this year. These innovative partnerships will allow us to stand alongside pharma companies leading the industry in improved patient […]

Upcoming PharmaVOICE Panel – Next Gen Patient Services Models
With increasingly less face-to-face patient interaction, manufacturers need to rethink traditional solutions in order to alleviate access barriers. Despite best efforts, prescription rates continue to drop as newly launched products experience increased patient drop-off; and companies are struggling to support patient acquisition, retention and conversion. In a new 60-minute virtual panel, PharmaVOICE Co-Founder and Editor […]

A Paul Simms Favorite: EVERSANA makes the list for 2021 Predictions
“Imagine there was a third way … an equivalent to the Apple App Store in our industry … maybe that model could start working in pharma; and, in fact, it is.” For pharmaceutical influencer Paul Simms, commercialization options for biotech and pharma companies aren’t great — in fact, they’re slowing the market down. In 2021, […]

Transforming Commercialization With Industry-Leading Partnerships
Healthcare innovation is being intensified as the industry rushes to find solutions to the pandemic, while furthering research in rare and complex diseases. As pharma companies reignite clinical development, streamlining commercialization is crucial in bridging the gap between treatment manufacturing and patient access. Recognizing this industry need, our team is actively partnering with companies leading […]
The 10-Year Test: Is It Possible to Plan Launch During Clinical Development?
Time and strategy are keys to launching in an overwhelmed, unpredictable market. Pharmaceutical companies in mid-development of a drug need to look ahead at their launch strategy options and consider what market conditions could look like by the time they’re ready to commercialize their product. By evaluating the value of an asset early in development, pharma companies can gain a better understanding of a product’s value and risk. With assessment insights, manufacturers can make informed clinical and commercial […]

Moving Forward in Pharma: Reigniting Revenue for Mature Brands
The world will be moving forward with immeasurable effects from the COVID-19 pandemic, as will the pharmaceutical industry. Fully understanding the impact that the pandemic will have on pharma will take years; however, there are future indications emerging for mature brands. Mature brands, or non-promoted in-line brands, are the bread-and-butter products for pharma companies in […]

How Better Alignment Propels Your Brand’s Success
As pharma companies race to keep up with today’s market, your brand’s team can’t afford to be out of sync. Yet inefficiencies and miscommunications are common throughout the commercialization process — wasting valuable resources, creating confusion among key stakeholders and slowing progress toward your brand goals. For many brand teams, these problems are often triggered by a lack of alignment […]

Emerging 2021 Life Science Priorities
From commercializing high-science therapies to empowering a data-driven patient experience (in the midst of a global pandemic!), we’ve learned that the life sciences industry requires agility, speed and scale. How will 2020 experiences set the emerging trends of 2021? Read below to learn more: Predictive Analytics The implementation of data and analytics has presented many […]

EVERSANA is Fighting COVID-19 Through a Growing Number of Client Engagements
The new year began with a light of hope ignited by the long-awaited COVID-19 vaccine. However, a vaccine is only the first step in fighting the pandemic. Healthcare professionals and drug manufacturers must continue to apply cutting-edge research and technology to overcome challenges related to vaccines, treatments and patient testing. While the industry’s top minds […]

PharmaVOICE Digital Influencers: Brigham Hyde
Data Is Transforming the Landscape In a special feature on Digital Influencers, PharmaVOICE Editor Taren Grom sat down with Brigham Hyde, President, Data & Analytics to discuss how data is transforming the life sciences landscape as we know it. From the rise of personalized medicine and value-based healthcare to evolving commercial strategies, the effective utilization […]

How to Enhance Provider and Patient Engagement in Tech-Savvy Healthcare
Pharmaceutical representatives have always been a welcomed resource and consistent office visitor for healthcare providers (HCPs). Today, in a world operating largely virtually, pharma reps are re-strategizing HCP engagement in the same way providers are rethinking patient outreach. As telehealth intercepts in-person appointments and office visits with HCPs become limited, providers will have to determine […]

Leading the Way in Digital Health With Tech-Driven Clinical Pathways
Digital health technologies have become integral to patient care since the beginning of the COVID-19 pandemic and will remain essential healthcare tools. As many patients and healthcare providers are embracing digital health devices, clinical pathway developers have an opportunity to lead the way in digital technology implementation — if they choose to. Healthcare providers rely on clinical pathways to […]

Insights From Dreamforce 2020 Panel: End-To-End Patient Engagement
EVERSANA was proud to participate in this year’s brand-new session “End-To-End Patient Engagement: From Clinical Trials Through Patient Support Programs.“ Bhaskar Sambasivan, Chief Strategy Officer and President of Patient Services, provided valuable insight into the emerging industry trends that are driving digital transformation within life sciences. He explained how manufacturers can embrace innovative technology to […]

Insights From Trade & Channel Strategies Conference 2020
Now more than ever, pharma manufacturers need to break the cycle of supply chain complexity to better connect patients to therapy. On Tuesday, December 1, EVERSANA’s Scot Buchanan moderated the panel “Emerging and Future Trends in Channel Strategy, Distribution and 3PL” with these industry leaders: Rob Osborne, Vice President, Pharma Trade Relations Express Scripts, Accredo, CuraScript […]

DSCSA Guidelines – Best Practices for Compliance and Supply Chain Integration
On November 27, 2023, all drug manufacturers must participate in an electronic package-level traceability system, commonly known as the “interoperability” requirement. EVERSANA is equipped to help manufacturers navigate these new guidelines and apply best practices. Let’s work together to make sure that you stay in compliance with the FDA and meet the demands of your […]

EVERSANA Receives PM360 Innovations Award for Seamlessly Disrupting Commercialization
EVERSANA’s end-to-end commercialization solution is reimagining traditional pharmaceutical commercialization models for the first time by breaking down industry service silos with integrated approaches and advanced technologies. PM360‘s Annual Innovation Issue was established in 2011 to provide pharma with its first-ever guide to the life sciences industry’s latest advancements. In its December 2020 edition, the publication is recognizing EVERSANA COMPLETE COMMERCIALIZATION as an innovative strategy that is pushing pharma forward and achieving improved […]

Solving Hard Go-To-Market Challenges [Webinar]
How can looking outside the box help identify innovative approaches that achieve market objectives? Today, in a world where pharma is shifting away from primary–care blockbusters and embracing specialty–care products, looking outside the box is essential. Focusing on HCPs during a product launch is no longer the key to commercial success. Instead, pharma companies must adapt quickly, leveraging artificial intelligence and machine learning technologies to acquire […]

Case Study: Early-Stage Asset Valuation
The rewards of developing a new drug can be very attractive but they come with inherent risks. It is important to ensure that investment decisions on clinical development are made after examining the risk profile of the drug which depends on the therapy area and the nature as well as extent of unmet needs. EVERSANA […]

Leadership Briefing – The U.S. Pharmaceutical Market Outlook: The Path to Recovery and the New Normal
During a recent webinar panel, EVERSANA and Reuters gathered industry leaders to discuss the tough questions facing pharma, specifically in the wake of a new U.S. presidency. EVERSANA CEO Jim Lang; Joe Jimenez, Ex-Novartis CEO and CEO and Co-founder of Aditum Bio; and Kabir Nath, Senior Managing Director, Global Pharmaceutical Business, Otsuka Pharmaceutical Co., Ltd., […]

Five Biggest Data Challenges for Life Sciences
Data is transforming the competitive landscape in life sciences. From the rise of personalized medicine and value-based healthcare to evolving commercial strategies, the effective utilization of data is critical to solving for better patient outcomes. There are significant challenges, however, including siloed, messy data quality; slow legacy systems delivering fragmented insights; and the inability to […]

Five Things You Should Do to Ensure Your PSP Is Ready for Launch Before Your Brand Is
Your brand is preparing for launch, and it’s time to develop a strategy for your Patient Services Program (PSP). Even if you already have one or more PSPs in place for your company’s other brands, it is important to make sure you don’t incorporate a one-size-fits-all approach for your PSP. In order to develop a […]

Approaching Clinical Pathway Development as an Art
Much like any artform, creating a clinical pathway requires forethought, innovative design and strategic execution in the hands of the physician — except in the art of healthcare, the most important critic is the patient. While physicians try to perfect the art of patient care, the market realities of sky-rocketing costs and fewer resources influence […]

Managing Risks to Improve Health Outcomes: How to Move Population Health Forward in an Era of Uncertainty
At the Canadian Association for Population Therapeutics (CAPT) Annual Conference, Sumeet Singh, Senior Director, EVERSANA, delivered a presentation on pharmacoeconomic analysis and outcome-based agreements and what they mean for Canadian private payers. Watch his session below for answers to the following questions: Are pharmacoeconomic analyses relevant for private payers? Should pharmacoeconomic analyses for private payers […]

Four Ways Predictive Analytics Can Strengthen Your Commercialization Efforts
Over the past few years, artificial intelligence (AI) and machine learning (ML) have been trending topics in healthcare but are now being propelled to the forefront of clinical decision support and care delivery. Some organizations, however, have been slow to adopt the utilization of predictive analytics for a number of reasons. From wasted investments in […]

Optimizing the Performance of a PSP and Improving Outcomes For All Stakeholders
Patient Support Programs (PSP) have been proven to improve clinical and patient outcomes and help manage patient out-of-pocket cost and prescribed use. Particularly for specialty drugs, the services provided under the PSP umbrella not only help remove barriers to patient access, they provide healthcare providers (HCPs) with tools they can use to help patients better […]

ODRD Insights: Building an AI-Driven Data Ecosystem to Drive Commercial Activities
The market landscape, especially for rare diseases, is dynamic and presents a unique set of challenges for the life sciences industry. Unlike the case for broader disease states, most rare disease patients are not correctly diagnosed for an average of seven years due to misdiagnoses, patient data sparsity or ambiguous coding. To address these challenges, […]

The U.S. Pharma Market Outlook: Path to Recovery and the New Normal
With the U.S. presidential election days away and the continued impact of a pandemic, EVERSANA and Reuters gathered industry leaders to discuss what is to come. Watch as the panel discusses the tough questions facing pharma, the need for new commercialization models, and a mandate to better promote the value we collectively provide as an […]

NORD 2020 Insights: Entering a New Era
EVERSANA was a proud sponsor of the 2020 NORD Rare Diseases & Orphan Products Breakthrough Summit to advance the dialogue on ways to improve the lives of over 25 million Americans living with rare diseases. Our mission is to advance the drug development and commercialization of orphan drugs, amplified by today’s need to create better […]

MARKET ACCESS AND TREATMENT OUTCOMES: Shifting From the Volume of Data to the Value of Insights
From Volume to Value: “The best prescription is insightful knowledge.” Former Surgeon General of the United States Dr. C. Everett Koop once aphoristically said, “The best prescription is knowledge.” This thought permeates much of what drives market access, but it is missing the essential word of the sentence, and that is “Insightful” knowledge. Dr. Koop […]

Population Health Partnerships To Advance Value-Based Care
The business model for health care in the United States is evolving from a volume-driven model to a consumer-centric, value-driven model. As such, there are new competencies required of hospitals and health systems to effectively manage a population’s health across the continuum of care. Many hospitals and health systems will need to partner with other […]

Mitigating Risk: When A Curveball Threatens Your Product’s Launch
In this 3-minute video, Mike DeLaroche shares a new commercialization model that mitigates risk and combats curveballs that manufacturers cannot predict when launching products.
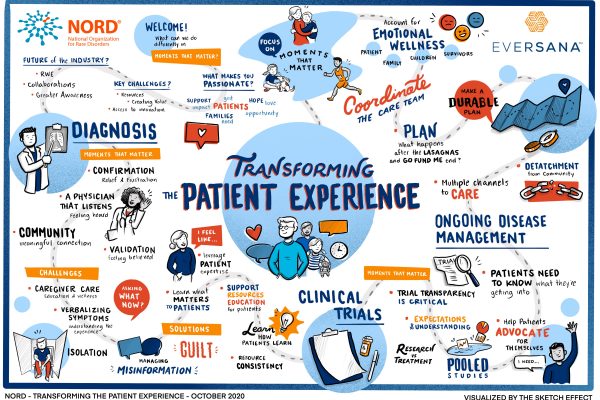
An Illustration: Transforming the Patient Experience
As a fierce ally of the rare disease community, EVERSANA proudly sponsored the 2020 Rare Diseases & Orphan Products Breakthrough Summit. Longtime partners in the advancement of orphan drug commercialization, EVERSANA and the National Organization for Rare Disorders (NORD) are dedicated to improving the lives of over 25 million Americans living with rare diseases. During […]

eBook – 2020 Stories That Shaped Commercialization
While many industries hit the pause button on their operations this year, the pharmaceutical industry never stopped, propelling new innovations to better serve patients, providers and stakeholders. At EVERSANA, we worked closely with manufacturers to tackle uncertain, complex market dynamics and solve pricing, access, reimbursement, adherence, and distribution challenges, and more. As we look forward […]

When A Curveball Threatens Your Product’s Launch
Download the article to learn about proven strategies to help shield your company from unwelcome surprises during launch by reducing your financial exposure and creating a more stable and flexible launch operation.

CBI Hub, SPP and eServices Insights: Leveraging Behavioral Health Technology
The Science of Human Resiliency — Behavioral Health Technology to Improve Patient Outcomes and Adherence Over the past several years, pharmaceutical manufacturers have been in pursuit of improving the patient experience by reducing time-to-treatment, developing a more integrated patient view and deriving insights that lead to better care. Additionally, physicians have been seeking new tools […]

PharmaVOICE Commercial Influencers: Greg Skalicky
In a special focus on Commercial Influencers, PharmaVOICE Editor Taren Grom sat down with EVERSANA’s Chief Revenue Officer Greg Skalicky to discuss approaches to commercial strategies and how disruptive companies are changing the market to better reach and engage patients. The conversation covered commercialization trends, how the industry has changed in the last few years, what is driving innovation within solution providers and small- to mid-size pharmaceutical […]

Compliance – The Competitive Differentiator to Commercialization: An Integrated Model Setting the New Global Standard
The Compliance experts at EVERSANA prove how integrated life science compliance has earned its seat at the table by demonstrating the value of increasing data-driven and technology-infused competitiveness in the successful commercialization of a new-age biopharma product.

WODC Insights: Developing HEOR Programs for Orphan Drugs
To successful launch a new orphan drug, demonstration of therapeutic success is crucial for a global HTA submission and securing market access. Manufacturers need to create a compelling value story with real-world data to illustrate how the therapy positively impacts the patient journey. In his informative presentation from the World Orphan Drug Congress 2020, Chris […]

NEXT GENERATION COMMERCIALIZATION: A CONVERSATION WITH THE INNOVATORS
Sell, out-license or launch internally: these are the three traditional options to commercialize a pharmaceutical product. Selling and out-licensing are common but cause innovators to lose ownership in an investment that takes years to develop. Launching internally requires an average investment of $125MM+ before the product even hits the shelves. Until now, there was no […]

WODC 2020 Insights: Leveraging AI/ML to Advance Rare Diseases
Leveling the playing field The average time to correctly diagnose a patient with a rare disease is 7 years, largely due to data sparsity, misdiagnoses, incorrect prescriptions, etc. Now, through the application of artificial intelligence and machine learning algorithms, we are able to shorten the time to diagnoses of rare diseases and even discover undiagnosed […]

Navigate the Complexity of Proposed CMS Medicaid Rule
In this presentation at MDRP’s virtual summit, Mike Kurland discusses several key regulatory proposals and executive orders and the impact on pharma: Operationalizing Value-Based Contracting – Has the door been opened, and how do we prepare for an increase in value-based strategies? Managing Coupons and Vouchers – Do we have the right data, and what […]

How to Get Your Digital Therapeutic Approved & Reimbursed in Europe and the U.S.
Patients, providers, and payers expect healthcare to be more accessible, intuitive, and adaptable to their needs. Barriers that have held back innovation in Digital Medicine and Telemedicine have been blasted apart with the recent relaxing of CMS and HHS requirements, continuing advances within the FDA’s Digital Health program, and additional reimbursement opportunities emerging globally. Get […]

PharmaVOICE100 Recognizes Two EVERSANA Leaders
For the past 15 years, PharmaVOICE has celebrated leaders throughout the life sciences industry who provide inspiration to their peers, colleagues and companies through their innovative and motivational approaches to addressing the industry’s myriad challenges. As defined by PharmaVOICE these individuals: set industry trends and create opportunities out of obstacles; are innovative and have the […]

WODC 2020 Insights: Innovative Value and Access Strategies
Innovative rare disease therapies deserve innovative value and access strategies. With over 1,500 attendees from 50+ countries, the World Orphan Drug Congress featured four days of education, conversation and brainstorming dedicated to drug development and commercialization. EVERSANA’s CEO, Jim Lang, had the esteemed honor of hosting Pfizer’s Vice President of Rare Disease, Patient & Health […]

The New Gold Standard of Drug Commercialization
Manufacturers spend >$125MM over three years leading up to launch, yet 66% of drugs don’t meet launch expectations. An unpredictable landscape, coupled with inevitable industry pressures, is forcing manufacturers to seek a more complete commercialization approach with less risk and more value.

The Importance of Digital Strategy in Pharma in the APAC Region
The Asia Pacific region continues to show great progress in digital health as pharmaceutical companies look at digital technologies as a tool to drive access and to start developing their digital strategies. To understand the strides and recent evolution of digital in pharma, our APAC team conducted a survey with top pharmaceutical companies in the […]

Improving Patient Outcomes Through Digital Health
Digital applications can work cohesively with therapeutics in the treatment of a broad range of diseases, from behavioral health to chronic conditions. In an interview with Debraj Dasgupta, Head of Strategy & Go-to-Market Planning at Nippon Boehringer Ingelheim Co., Ltd., Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, […]

The Digitalization of Healthcare in Asia Pacific
The digitalization of healthcare in Asia Pacific is being utilized to enhance the patient-centered approach and expanding access to health. In a candid conversation with Abhishek Shah, CEO at Wellthy Therapeutics, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, uncovered the current healthcare landscape and how digital strategy […]

The Regulatory Landscape in Digital Health
Despite the advances in digital health and digital therapeutics in recent years, there remains an ambiguity in the digital space and uncertainty surrounding regulation and reimbursement of digital technologies applied to pharma, biopharma and medical device companies. In an insightful conversation that was part of EVERSANA’s Digital Symposium, Ed Cox, Executive Vice President, Strategic Alliances […]

Access to Technology and the Rise of Digital Health in Asia Pacific
The access to technology, development of telehealth and evolution of digital therapeutics are thriving in Asia Pacific; and pharmaceutical companies are levering the momentum to reach to a broader segment of the patient population. Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, interviewed Anish Shindore, Head of Digital […]

Digital Innovation in Asia Pacific
Digital medicine and digital therapeutics are transforming the healthcare industry by changing the care delivery format, and they have the potential to improve patient adherence and outcomes. In our recent Digital Symposium, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, interviewed David Keene, CEO at IntroSpect Digital Therapeutics […]

Outlook: Digital & Pharma in Asia Pacific
Asia Pacific is one of the fastest-growing regions for digital innovation in the pharmaceutical and biotechnology industries. Its promising outlook is mainly driven by necessity, commercial flexibility and a broad talent pool. In a candid conversation broadcasted during the EVERSANA’s Digital Symposium, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine, […]

Copay Accumulator & Maximizer Programs Are Changing
A Look at Copay Assistance Programs and Copay Accumulators Copay assistance programs are a known tactic deployed by drug manufacturers to help facilitate access to often expensive specialty medications. These financial assistance programs take a comprehensive approach to supporting a patient’s out-of-pocket costs, inclusive of any applicable deductibles and drug cost-share responsibilities – up to […]

Insights from ISPOR 2020
Shaping the future of patient care. From a panel discussion to podium and workshop presentations, EVERSANA shared insight at this year’s virtual ISPOR 2020 conference. More than 1,300 attended the conference from 54 countries; and our experts demonstrated the impact of comprehensive HEOR capabilities, including economic modeling, evidence synthesis, value communication and reimbursement strategies, across […]

Ask the Expert: Digital in Pharma | The Critical Questions
EVERSANA is uniquely positioned to follow the trends and introduce new models and strategies to maximize the value of pharmaceutical products through Digital Medicine Solutions and Execution. Digital Solutions can be deployed to impact the entire life cycle of a product in unprecedented ways. In a “digital tell-all” conversation, Ed Cox addresses four critical questions […]

Podcast: The Future of Digital Therapeutics
The Digital Therapeutics market continues to evolve at a rapid pace. In this podcast, hosted by Erica Sosnowski, host of the podcast series “The Platform”, we discuss current trends in the life sciences industry and the future of digital therapeutics with Edward Cox, the Global Head of Digital Medicine and Executive VP of Strategic Alliances […]

A Predictive Analytics and Machine Learning Approach to Improving Hub Performance and Patient Outcomes
Today we have access to more data, from more sources than we could ever dream possible. Living in a digital world, we increasingly need the ability to efficiently and effectively process this data for insights and actions in order to be competitive. The life sciences industry can leverage this data using analytic tools and machine learning to rapidly identify patient behaviors and patterns – allowing us to predict “next best actions” in our quest to improve patient outcomes.

Leading and Decision-Making in Uncertain Times
By definition, leaders must have followers, who are defined as people who must be shown the way. While this is within most leaders’ grasps when the road ahead is straight and wide and the destination is visible, more is demanded of them when the road splits, narrows and dusk falls. The current crisis presents special […]

5 Trends in Digital Medicine to Watch For
We Are Uniquely Positioned To Deliver EVERSANA is uniquely positioned to follow the trends and introduce new models and strategies to maximize the value of pharmaceutical products through Digital Medicine Solutions and Execution. Digital Solutions can be deployed to impact the entire life cycle of a product in unprecedented ways. Schedule a 30-minute call […]

Healthcare Pivot: Digital Medicine Delivers During A Global Crisis – Reviewing Commercial Pathways
In February 2020, healthcare as we knew it changed. The industry began waging an aggressive war with an unknown virus beginning to overwhelm hospitals on a global scale. We immediately recognized that we needed to do things differently. Virtual healthcare began to rise, and disruptive advancements in medical technology began breaking down siloed healthcare delivery […]

PharmaVOICE Precision Medicine Showcase
Improving Patient Adherence With Behavioral Health Technology The industry’s shift to outcomes-based, patient-centered care requires new approaches to patient support to achieve adherence and therapeutic success. Today’s patients are also highly motivated information seekers and are taking control of their day-today therapy. And, like all modern consumers, they expect outcomes to be personalized, delivered on […]

Two Key Questions About Payer Strategies in 2020
In a PM360 Focus on Payer Strategies feature, Brian Davis addresses the question: Besides efforts to lower drug prices and increase transparency, what market access challenges/barriers will cause pharma companies the most stress this year? How can companies overcome this challenge/barrier? In his response, Brian highlights a few of the 2020 challenges to market access, […]

PharmaVOICE Showcase on Connected Health and IoT
The Growing Potential of Connected IoT In PharmaVOICE’s Showcase on Connected Health and IoT, Ed Cox offers executive viewpoints on the challenges of integrating the Internet of Medical Things (IoMT) into pharma processes. “Breaking down siloes by supporting technology that provides value across the patient care ecosystem and investing in IoMT that generates data and […]

PharmaVOICE Outsourcing Showcase
The New Roaring 20s: How Outsourced Commercialization is the Revolution We Need. To effectively commercialize a product, traditional service silos stand in the way of true healthcare transformation. Do disparate service providers look at the life cycle in the same way you do? There is a real need for a market access strategy that converts […]

A Patient Hero
Making the Patient an Extension of the Team The concept of patient-centricity is well embedded throughout EVERSANA and is intrinsically tied to helping the agency’s clients achieve their goals. Kristin LaBounty Phillips believes that the entire agency — with a unified commitment to quality in addressing patients’ needs — embodies the concept of “patient hero.” […]
Realizing Value from Digital in the Healthcare Environment [Slides]
[Presentation Slides] The advancements of Digital Therapies are promising but commercial success is not always guaranteed. This presentation explores the pitfalls and challenges of selling products into the uniquely complicated and regulated healthcare market. It will lay the groundwork for digital companies to have successful commercial strategies and be able to: Answer questions like: What […]

Realizing Value from Digital in the Healthcare Environment [Webinar]
[WEBINAR – 20 MINUTES] The advancements of Digital Therapies are promising but commercial success is not always guaranteed. This presentation explores the pitfalls and challenges of selling products into the uniquely complicated and regulated healthcare market. It will lay the groundwork for digital companies to have successful commercial strategies and be able to: Answer questions […]

The New Roaring 20s: How Outsourced Commercialization is the Revolution We Need
The “Great Gatsby Era” was an exciting time of new prosperity, infinite creativity and dramatic social change. Long-standing social norms and traditions gave way to the “mass culture” and consumerism that modernized American society. “What will they think of next?” was a popular expression that defined the era. On the 100-year anniversary of this revolutionary […]

Trends Disrupting Life Sciences in 2020
From the commercialization of digital medicines to the evolution of value-driven healthcare – our thought leaders deliver insight on the trends impacting the life science industry that will continue to shape our thinking well in 2020 and beyond. Take a look at the trends we’re tracking by clicking on the links below: The Transformative Promise […]

How to Maximize the Value of New Advances in Oncology
Today we have access to more data, from more sources than we could ever dream possible. Living in a digital world, we increasingly need the ability to efficiently and effectively process this data for insights and actions in order to be competitive. The life sciences industry can leverage this data using analytic tools and machine learning to rapidly identify patient behaviors and patterns – allowing us to predict “next best actions” in our quest to improve patient outcomes.

Strategies to Ensure Market Success of a Rare Disease Product Launch
Small patient populations, complex administration, high costs of therapies, and government policy interventions are just a few obstacles in rare disease. EVERSANA’s Managing Director, Bill O’Bryon, and Senior Director, Diann Johnson, outline key insights to consider in your launch strategy: Market dynamics impacting rare disease therapies The entire rare community: patients, caregivers, providers & advocates […]

Ask the Expert – Digital Therapeutics
From reimbursement strategies, distribution, dispensing, patient engagement and adherence programs, EVERSANA offers integrated solutions to alleviate the barriers facing the adoption of digital therapeutics. Mike Ryan, Executive Vice President of Europe and Asia Pacific, discusses how EVERSANA is leading the commercialization of digital therapeutics with an innovative and nimble commercial model valuable to patients, providers […]

WHAT IF… We Broke the Silos Throughout Pre- to Post-Commercialization?
In their special November/December issue, PharmaVOICE asked industry leaders to think beyond the status quo and provide insight into what needs to happen to achieve aspirational goals and evolve the healthcare industry in 2020. In the feature article titled, What if…” PharmaVOICE turns to their community of thought leaders to weigh in on their own […]

After FDA Approval, Can Gene Therapies Achieve Marketing Success?
Without a doubt, gene therapy is transforming healthcare by revolutionizing patient care from conventional treatment models to curative therapy models. There are more than 7,000 distinct types of rare and genetic diseases and 400+ million individuals suffering from a rare disease. With a market that is fragmented, there is a need for an innovative, end-to-end […]

Innovative Therapies Call for an Integrated Drug Safety and Compliance Model
In this article, Herbert Lee, PharmD, Senior Vice President, Medical Communications and Pharmacovigilance, makes the case for an integrated approach to ensuring the safe and effective use of medications by patients. From emerging therapies to innovative technologies, the healthcare industry is changing – demanding that we do business differently. Working in one of the most […]

Building a Service Offering from the Research Lab to the Patient’s Home
A Conversation with Pharmaceutical Commerce & Jim Lang, Chief Executive Officer, EVERSANA Not quite a year ago, an assemblage of pharma service providers came into being as EVERSANA. Funded by two private equity firms with a background in healthcare—Water Street Healthcare Partners and JLL Partners, there were six acquisitions at the time: Dohmen Life Science […]

Are We Transforming In The Right Way?
Why Product Launches Can’t Be Distracted By Empty Promises. We can all agree that innovations in therapeutic development have advanced beyond traditional product launch strategies and service models. In every step of the product lifecycle, we see pockets of transformation. The problem is exactly that – “pockets” of transformation. Your product launch strategy has to […]

Four Key Questions About Patient Experience in 2019
In this September feature, PM360 asked experts for insight into the changes that need to happen to create better experiences for patients and ultimately serve them better. Kathi Henson, Chief Patient Service Officer, shares insight into how the increased access to real-time patient data helps life sciences companies better understand patients and improve their experiences. […]

EVERSANA, Patient Experience Project featured in MM&M July Issue
Each year, Medical Marketing & Media compiles a list of the top 100 medical marketing agencies in North America. As MM&M Editor Stephen Madden shares at the opening of the July issue, “A typical issue of MM&M has 56 pages; with 244, this beast has almost five times as many.” From agencies A to Z, […]

Gene Therapy for Rare Disorders
With only three approved products in the marketplace, how do we build a regenerative medicine ecosystem that delivers more value to patient’s faster?

Setting a Patient Engagement Framework and Proving Value
Is your culture patient-centric? It needs to be because today’s patients are motivated information seekers – empowered decision-makers with respect to their treatment. Their voice is key to your success.

Insights from AMCP 2019
EVERSANA attended (and sponsored) The Academy of Managed Care Pharmacy (AMCP) Managed Care & Specialty Pharmacy Annual Meeting. If you missed the conference, here are a few key takeaways from our colleagues who attended: 1) Federal and State Legislature Update Increases in federal and state policies are putting more pressure on drug prices The possible removal […]

Providing a Patient-Driven Experience
As the “patient-as-consumer” culture enables patients to consider all their varied care options, they increasingly want to be involved not only by participating in clinical trials, but also by accelerating product development and the availability of patient support programs after a product reaches the market for a rare and complex disease. And, like all modern […]

Sell’N Gene Therapies: A Landscape Assessment Of Cell And Gene Therapy Reimbursement
Cell and gene therapies hold great life-saving potential, with the ability to treat disease states with significant morbidity, mortality, or treatment-related complications. Kymriah became the first CAR-T cell therapy to bring transformative efficacy to B-cell acute lymphoblastic leukemia in children and young adults in August 2017. Luxturna—the first-ever treatment for a rare form of hereditary […]

Put Your Patient Services Program to the Test
How well do you really know your patients? Global Genes reports that therapy adherence in the rare disease space can vary from 58 to 65 percent, a troubling statistic for our industry, and most importantly, the patients we serve. Manufacturers must be wondering, how well do we really know our patients and what they need? […]

Will the U.S. adopt global reference pricing?
This article was written for Med Ad News Magazine There are many things that Americans get from outside our U.S. borders. Our top imports include oil, machines, and cars; our iTunes and Spotify accounts are stocked with The Beatles, U2, and Ed Sheeran; and you practically can’t go to any city in America without being […]

Product Master Data Management
This white paper shares considerations, common pitfalls and key takeaways for manufacturers participating in Medicaid. Recent market and enforcement trends in healthcare further exemplify the transition from a volume to value-based marketplace, as well as the complexities to contain the increasing costs of drugs in the new paradigm. The costs of branded prescription drugs are rising […]

The Radical Implications Of Indication-Specific Pricing
This paper discusses one of the potentially more impactful new dynamics in the pricing of pharmaceuticals—the advent of indication-specific pricing. Our goal is to provide context to this important change, highlight some of the implications about which biopharma companies should be aware, and leave you with a framework for raising and addressing pertinent questions across […]

Don’t Poke The Bear: The Effect Of Pharmaceutical Pricing On Perception And Future Innovation
Companies like Turing Pharmaceuticals and KV Pharmaceuticals have grabbed headlines recently for their aggressive pricing strategies. Whether these strategies are justified or even successful is not the point. Rather, what is important is the negative public perception that significant price hikes create for the industry as a whole. In this climate, the industry needs to […]

