Medical Information Market Landscape
As technology advances and the needs of healthcare professionals and patients change, the Medical Information (MI) market dynamic must evolve in tandem.
Most MI departments have transitioned from in-house contact centers to leveraging a contact center(s) operated by a service provider(s). The traditional call center model is outdated and centers of excellence for customer experience supersede them. In addition, MI services have gone beyond call centers, email, online web request forms and self-service portals for healthcare professionals, to now providing medical self-service portals for patients and consumers, offering live chat with medical information specialists, and building chatbots to address the most received questions. MI departments must ensure the content provided is easy to digest, navigate and utilize for different customer types.
Now more than ever, partnering with the right MI service provider is crucial to ensure customer satisfaction and that appropriate service levels are met.
EVERSANA’s Next Gen Medical Information Model
EVERSANA’s holistic and customer-centric approach to MI services reimagines the industry’s one-size-fits-all model with an end-to-end, global solution tailored to each client’s needs with a focus on quality, value and expertise across people, process and technology.
We form strategic and collaborative partnerships with our clients to develop tailored, flexible and scalable resource models, based on their needs, therapeutic areas, products, operations and infrastructure.
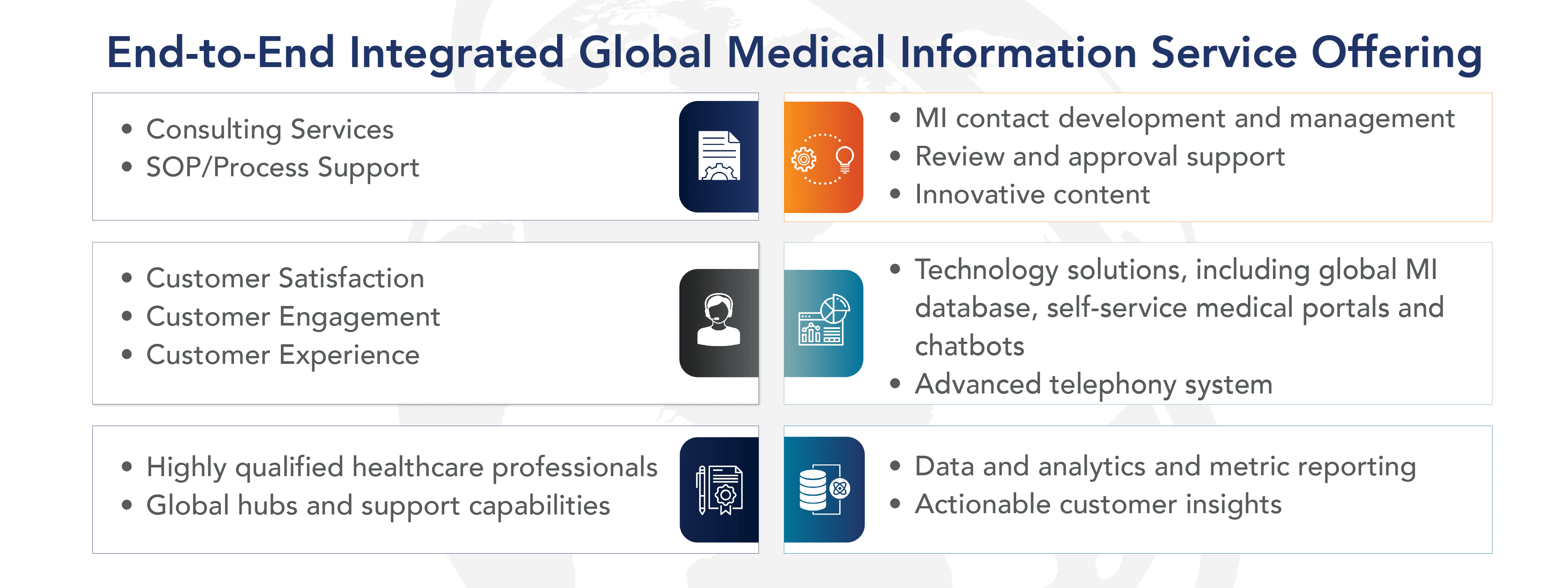
EVERSANA offers one-stop services for organizations looking to establish and launch their MI services for the first time. These services include:
- Consulting services regarding industry trends and best practices; medical content development (e.g., SRDs, FAQs)
- Technology solutions such as validated and configurable MI database
- Self-service medical portals
- Medical chatbot with conversational AI and data analytic tools
- Global hubs with highly trained MI Specialists that are healthcare professionals (e.g., pharmacists)
Foundational Pillars of EVERSANA’s Medical Information Services
EVERSANA’s four foundational pillars of our MI Services provide high-quality service and engagement throughout the product life cycle and ensure optimal support of our clients’ stakeholders. Our expertise across people, process and technology has allowed us to establish operational excellence.
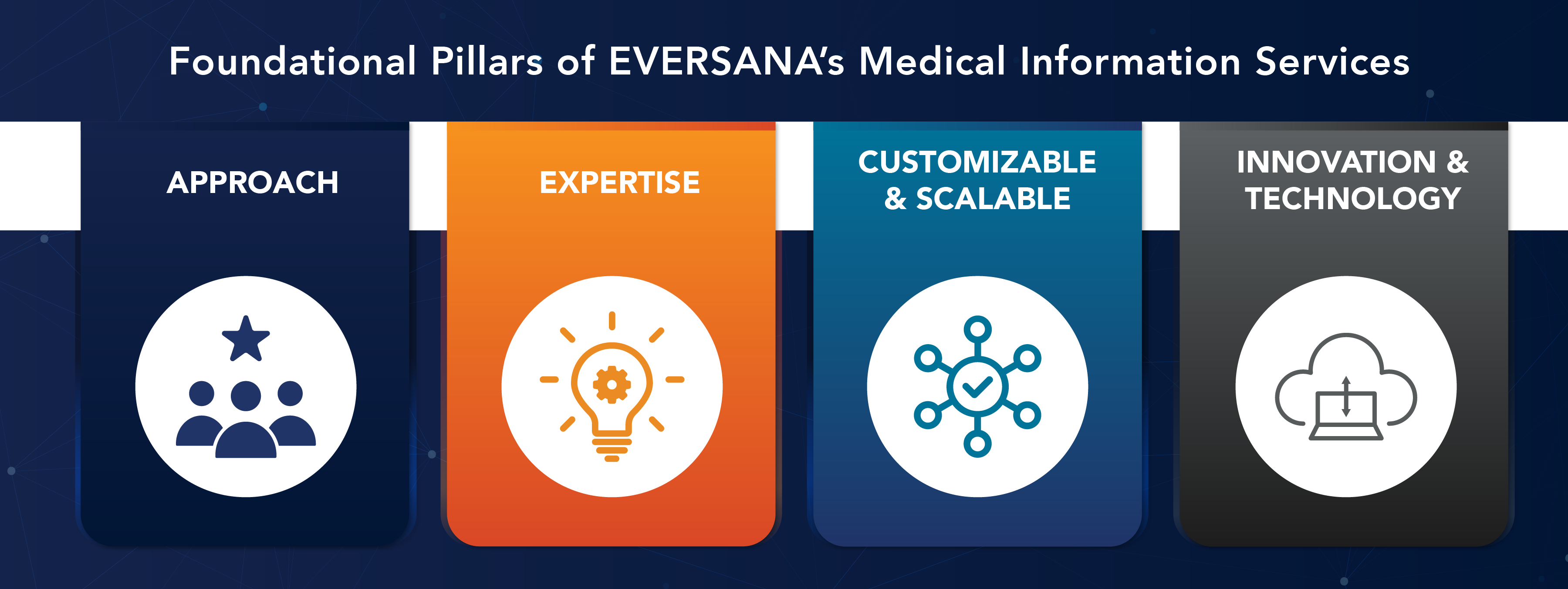
- Our approach is Value and Quality driven and deep-seated in our cultural beliefs of Client Delight and being Patient-Minded
- 20+ years of MI services experience supporting 200+ clients and extensive disease state expertise across multiple therapeutic areas
- EVERSANA develops tailored, flexible and scalable resource models to ensure key performance indicators and first-rate customer satisfaction and engagement are met
- Our MI services are deeply rooted in technology, innovation, quality, compliance and industry best practices, featuring multi-channel engagement, and are backed by metrics and data analytics
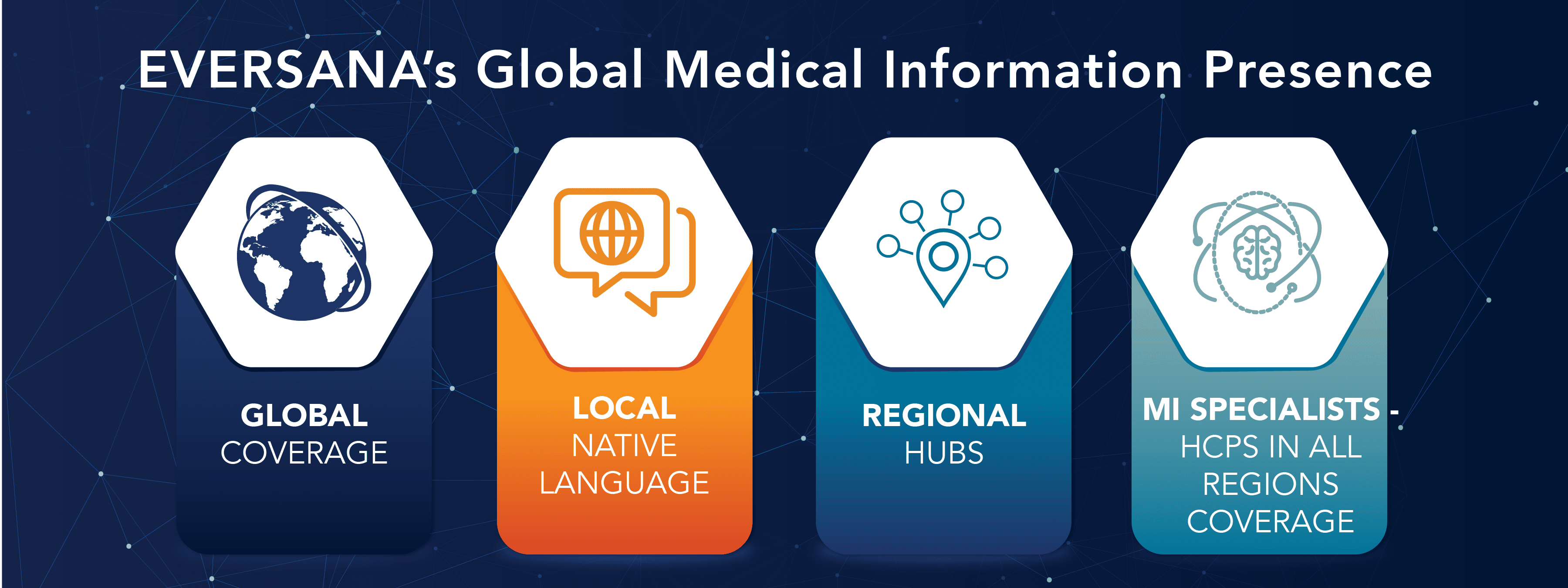
Global Capabilities
With established global MI hubs in the U.S., Europe and India, EVERSANA provides MI support globally to clients in the U.S., Canada, Europe, LATAM and APAC. EVERSANA’s global infrastructure will soon expand hubs and services to Japan and China.
EVERSANA hires and trains MI Specialists, primarily pharmacists, with healthcare professional backgrounds across all our hubs to manage medical inquiries.
EVERSANA’s global MI capabilities include:
- Native language support from our locally based medical information teams
- Understanding of local cultures, laws, regulations and best practices in relation to medical information inquiry, adverse events and product complaint handling
- Flexible staffing models (Shared, Dedicated and Hybrid) and scalable global contact center solutions to meet your changing needs
Ready to talk to medical information experts about how this model can support your strategy and tactics? Schedule a meeting.

