
EVERSANA Team
EVERSANA employs a team of over 6000 professionals across 20+ locations around the world. From industry-leading patient service and adherence support to global pricing and revenue management, our team informs the strategies that matter the most to our clients and perform superior services that create value across the product life cycle.
Articles by EVERSANA Team

Navigating Successful Partnerships in the Pharmaceutical Industry: Insights from Shorla Oncology CEO Sharon Cunningham
In the ever-evolving landscape of the pharmaceutical industry, forging successful partnerships and collaborations is a cornerstone of achieving groundbreaking advancements and bringing innovative treatments to patients in need. However, navigating through the myriad of advisors, consultants and potential collaborators can be daunting for even the most seasoned professionals. Enter Sharon Cunningham, CEO and Co-Founder of Shorla Oncology, a visionary leader who understands the importance of strategic partnerships in propelling Shorla’s mission forward. Sharon shares invaluable insights gleaned from her experience, shedding light on the key factors that contribute to successful collaborations in the pharmaceutical arena. One paramount consideration highlighted by Sharon is the alignment of values and the presence of […]

Measuring Willingness to Pay: The Next Access Frontier
EVERSANA’s APAC Team Authors: Robin Arnold, Swapnil Waichale, Lakshmi Pragna Kalavacharla, Utkarsh Sahu Patient access to expensive healthcare poses a significant challenge in the pharmaceutical industry. As the cost of developing innovative medicines rises, the demand for accessibility as a basic human right increases. This POV explores the evolution of Patient Access Programs (PAPs) from financial to value-based models, emphasizing the need to address patient willingness to pay for treatments. Despite financial accessibility assurances, some patients still hesitate due to various factors, prompting the need to measure willingness to pay independently of financial status. This POV delves into the complexities of assessing willingness to pay and highlights the lack of […]

CES 2024 Recap – How the Future of Healthcare Will Continue to Be Driven By Innovation & AI
CES 2024, the annual tech conference encompassing everything from cutting-edge gadgets to automotive marvels, healthcare innovations, and futuristic media, left a lasting impression. Amidst the buzz surrounding electric vehicles, transparent screens, and an abundance of massage chairs (so many massage chairs), there was one technology that overlapped across all: AI. The rapid integration of AI into all facets of the showcased technology emerged as a standout theme, especially in the life sciences space. Beyond the mere mention for hype, practical and impactful AI stole the spotlight, becoming a necessary tool for delivering on product promises and enabling insights, new interactions, and even new digital worlds. Consider these three key takeaways. […]
Impact of the European Union Medical Device Regulation Extension on Legacy Devices
Question: The European Union (EU) has extended the transition period for medical devices under the Medical Device Regulation (MDR) and removed the sell-off provisions for existing products. How do these changes impact you? Answer: On 7 March 2023, the European Council voted to adopt a measure to extend the transitional period for medical devices under the European Union Medical Device Regulation (MDR). The extension is intended to address the risk of shortages of critical medical devices due to low notified body capacity. The extension provides manufacturers and notified bodies alike with added breathing room to effectively transition to MDR from current MDD Certificates. The extension applies to legacy devices of […]

The Hospital of the Future and How Data, Digital and AI Merge to Make Care Better
By Pierantonio Russo, MD, FCPP, FAAP, STS, Chief Medical Officer, EVERSANA In the fast-paced realm of healthcare, innovation stands as the cornerstone of progress. Recently, I had the privilege to attend Frontier’s Health , a leading gathering of some of the best minds in the world to discuss how to shape the future of health care. I was honored to take center stage and share some insights on what the health care delivery system and what the ‘Hospital of the Future’ might look like. There are seismic shifts occurring in the healthcare landscape today. Currently, the convergence of cutting-edge technologies, availability of large amount of data from multiple sources, increasing […]

Robotics in Healthcare: Revolutionizing Patient Care
Authors: Robin Arnold, Ankit Kulshrestha, Abheek Bose, Mayur Muley, Anant Kasodekar The launch of the PUMA 650 surgical robot in the 1980s, initially employed in brain biopsy procedures, ushered in the era of medical robots.1 From then until the present day, remarkable innovations have led to the development of state-of-the-art robots and their seamless integration into diagnosis, surgery, and rehabilitation. These innovations were driven by the shortage of skilled healthcare professionals to meet the growing demand for minimally invasive procedures, and the need to enhance patient outcomes. The global medical robotics market is poised to grow by 21.3%, reaching $35 billion by 2030.2 This robust growth stands testament to the […]

The Data-Driven Revolution: AI’s Impact on Pharmaceutical Quality in Digital Therapy
In the intricate web of pharmaceuticals, a groundbreaking transformation is underway – one that marries data analytics with digital therapy, reshaping the industry’s landscape fundamentally. As the dust settles from the FDA Symposium, a seismic shift in focus becomes apparent: the integration of Artificial Intelligence (AI) in Digital Therapy isn’t just a trend; it’s a data-driven revolution with quantifiable impacts. Let’s dive into the numbers. Recent market trend analyses reveal a sharp uptick in AI adoption within digital therapy. In 2022 alone, the global digital therapy market saw a 42% increase in AI-powered solutions, a figure projected to double by 2025. These statistics underscore a crucial point: pharmaceutical companies are […]

Navigating the Evolving Landscape of Internet Healthcare in China: Development Status, Challenges, and Future Prospects
Authors: Robin Arnold, Executive Vice President; Renyang Liu, Engagement Manager; Erica Wang, Associate Consultant Background Internet healthcare means using the internet to provide health and medical services. China has implemented “Internet Plus Healthcare” as a national policy, as a part of reforming the healthcare system. Internet healthcare in China is estimated at 150 billion RMB in 2023, with various applications, including online diagnosis, online medical consultation, online sales of pharmaceuticals, and “IoT” mobile medical devices. Internet healthcare is evolving and expanding due to increasing demand and technological innovation. Complete the form below to download the full article.

Unveiling the Future: AI Opportunities in FDA Compliance, Insights from the OPQ Symposium
In the dynamic realm of pharmaceuticals, staying ahead of the curve is not just an advantage; it’s a necessity. Recently, our team had the privilege of attending the CDER’s Office of Pharmaceutical Quality (OPQ) symposium, where the focus was on a topic that’s transforming the industry: Artificial Intelligence (AI). As consultants deeply involved in FDA compliance, this topic piqued our interest and left us with profound insights into the hurdles and opportunities that lie ahead. The symposium buzzed with discussions about the AI challenges faced in ensuring FDA compliance. One of the key points highlighted was the need for AI algorithms to align seamlessly with the stringent regulatory frameworks. As […]

Driving Patient-First Affordability in Seconds – Not Days: Accelerating Time to Therapy
Fewer than 60% of Pharmacy Benefit Manager (PBM) electronic benefit verifications (eBVs) accurately assess patients’ financial responsibilities, largely due to the industry’s reliance on AI algorithms for estimations. This leads to financial barriers for patients, hesitancy among healthcare providers (HCPs) to prescribe therapy, and increased burdens on Patient Assistance Programs (PAP) and Hubs. In this webinar hosted by Pharmaceutical Executive, EVERSANA experts Kevin O’Meara, Matt Baniak and Jeff LaVaute are joined by a guest speaker as they discuss issues facing eBVs and how an innovative approach that includes a system of product archetypes, proprietary software, automated business logic, and strategic payer relationships can improve price transparency and provide stakeholders access […]

Making Primary Market Research SMARTER Through Social Listening
Discover how EVERSANA is transforming the landscape of primary market research in the pharmaceutical industry through our unique approach to social listening. This insightful article from our APAC team highlights the common challenges faced by pharma companies and how most fall short in translating social insights into actionable strategies. EVERSANA, on the other hand, stands out by making social listening truly actionable. Learn how we seamlessly integrate social insights into primary market research, creating a powerful synergy that delivers tangible results for their clients. Don’t miss the opportunity to gain valuable insights into this game-changing approach – download and read the full article now! Complete the form below to download […]

APACMed Digital Health Reimbursement Policy Forum
Introduction The APACMed Policy Forum on Digital Health Reimbursement was held on 23 May 2023, with participants from Australia, Japan, Singapore, South Korea, Germany, France, and the UK. Several digital health policymakers, academicians, and experts from the respective countries, along with the APACMed Digital Health Reimbursement Alliance (DHRA) core committee, local trade associations, and EVERSANA members participated in the event. The forum was structured across three sessions: Session 1: Background on the digital health landscape across APAC, US, and Europe Session 2: Key learnings from APAC and Europe in the digital health reimbursement area Session 3: Scenario-based case discussion on an imaginary digital therapeutic product The policymakers shared the current […]

New Models of Benefits Verification Solve for Complexity, Accelerate Speed to Therapy and Deliver Price Transparency
Navigating health care insurance coverage can be a supremely convoluted process for anyone, especially patients or caregivers recently faced with coping with a life-threatening disease. While the advancement of many new treatments and scientific innovations in the form of “specialty drugs” has been invaluable in treating complex diseases, these conditions often muddy the benefit verification process even further by requiring complicated dosing regimens, burdensome administration methods and monitoring, and visits to different specialists. Additionally, there may be a multi-payer coverage landscape depending on a variety of factors such as administration route, commercial or government plan and accompanying requirements for therapy. Patient-First Access and Affordability in Seconds, Not Days The Patient […]
Unlocking Global Success: The Crucial Role of Integrated HEOR Programs in New Product Launches
Author: Kirk Szafranski, Director, HEOR, Value & Evidence Health Economics and Outcomes Research (HEOR) programs play a pivotal role in assessing a product’s value, cost-effectiveness, and real-world impact on patient outcomes, which are critical factors influencing reimbursement decisions, formulary placements, and overall market access. A global integrated HEOR program typically includes a range of components and activities, including but not limited to: Systematic Literature Reviews (SLRs): Comprehensive and systematic reviews of existing published literature related to the product, its therapeutic area, and relevant comparators. SLRs help gather evidence on clinical effectiveness, safety, and patient outcomes. Global Value Dossier (GVD) Development: The creation of a comprehensive document that outlines the product’s […]

The Rising Demand for Light Medical Aesthetic in China
“The Rising Demand for Light Medical Aesthetic in China” provides a captivating insight into the surging popularity of non-surgical medical aesthetics in China. The article highlights the rapid growth of the medical aesthetic market and the shifting trends in consumer preferences. It delves into the key contributors and experts driving this industry and examines the challenges posed by the pursuit of beauty, such as addiction and illegal procedures. The comprehensive analysis showcases the dominance of hyaluronic acid injection as the preferred choice among consumers and delves into its various applications and future trends. Furthermore, the article explores the potential of botulinum toxin injection and regenerative injections, shedding light on their […]

Revolutionizing Vaccines: The Rise of mRNA Technology in China
Key Contributors: Robin Arnold, Executive Vice President; Renyang Liu, Engagement Manager; Nicole Li, Analyst Discover how mRNA technology is transforming the field of vaccines in China. From the evolution of vaccine development to the rapid progress of mRNA vaccines during the COVID-19 pandemic, this article explores the advantages of mRNA vaccines compared to conventional ones. Delve into the efforts of Chinese pharmaceutical companies to develop mRNA vaccines, overcoming policy barriers and seeking international markets. Learn about the potential applications of mRNA vaccines in areas like cancer immunotherapy and infectious diseases. Don’t miss the opportunity to explore the future of vaccines and how China is striving to develop safer and more […]

Maximizing Potential: Essential Steps for Successful Commercialization of Cell and Gene Therapies
Introduction Cell and gene therapy (CGT) products have significantly enhanced the quality of life for millions of patients impacted by medical conditions that are untreatable with traditional medicines or surgeries such as certain cancers, inherited and rare diseases, and intractable conditions. As these therapies continue to propel groundbreaking advancements in remedying previously incurable diseases, the complexities of properly commercializing them and ensuring ample patient access can present significant challenges for biopharma manufacturers. The FDA and EMA are forecasted to approve and license approximately 30 new CGT products annually by 2025, and the global cell therapy market is projected to reach $247 billion by 2028. This creates tremendous potential for manufacturers, […]

The Art of Simplifying Pharmacovigilance – Part I: Connecting the Dots
The Art of Simplifying Pharmacovigilance – Part I: Connecting the Dots In the intricate realm of pharmacovigilance, there is an art — a delicate dance of connecting the dots. Like a skilled painter, vigilant professionals adeptly simplify the complex web of drug safety, linking scattered points to reveal a cohesive picture. With unwavering focus, they navigate vast databases and countless reports, tirelessly seeking patterns, correlations, and signals. Each connection brings a clearer understanding, empowering them to safeguard the health and well-being of countless individuals. It is a symphony of vigilance, where data harmonizes with expertise. The art of simplifying pharmacovigilance is a masterful tapestry of insight and diligence, painting a […]

Commercial Key Success Factors (KSFs) for Global Drug Development Programs
Key Contributors: Swapnil Waichale, Principal and Robin Arnold, Executive Vice President Discover the essential commercial key success Factors (KSFs) that drive triumph in global drug development programs. In an era of diverse regulatory perspectives and reimbursement complexities, biotech companies face higher stakes than ever before. Swapnil Waichale and Robin Arnold unveil the top failure modes for biotech firms and present a groundbreaking set of ten KSFs that can revolutionize the path to commercial success. From securing parallel consultations with regulatory and HTA authorities to optimizing country launch sequences, these strategies are the keys to unlocking global opportunities and maximizing profitability. Dive into this enlightening article and position your drug development […]

The Role of RWE in Expediting the Drug Approval Process
Key Contributors: Lydia Edison (Project Manager), Rohit Mandlik (Project Manager), Rohit Dang (Engagement Manager), Mahendra Rai (Senior Director) The role of real-world evidence (RWE) in expediting the drug approval process. RWE refers to the use of real-world data (RWD) from sources outside traditional clinical trials to support regulatory decision-making. RWE offers several benefits, including generating evidence sooner, optimizing clinical study design, providing data on long-term outcomes, including diverse patient populations, and improving decision-making for stakeholders. The use of RWE in drug approval processes has gained significant attention, with the US FDA establishing an RWE program to explore its potential. RWE-supported approvals have increased over the years, demonstrating its growing significance. […]

Specialty Pharmacies are Ready for the Biosimilar Boom. Are you?
The pharmacy benefit biologic market is primed for disruption, with Specialty Pharmacies (SPs) eagerly awaiting the pending market shift. While biosimilars have been available for nearly a decade in the United States, this year will be the first time many SPs begin dispensing these brands. Currently, 22 biosimilars are available in the U.S. and, up until recently, nearly all 17 of these brands fell within the therapeutic area of oncology/oncology support markets, where insurance coverage is typically billed through the medical benefit. Unlike many oncolytic biosimilars, several (if not all) of the newly available or soon-to-launch autoimmune biosimilars are self-administered products, which will largely be covered by the patient’s pharmacy […]

Position Your Future-Facing Strategy on Digital Therapeutics (DTx) in China
How can DTx resonate with current portfolios and capture incoming opportunities? Key Contributors: Robin Arnold, Executive Vice President; Jerry Song, Associate Principal; Qiwei (Alex) Li, Consultant The article discusses the potential of digital therapeutics (DTx) in China’s healthcare system and how it can help pharmaceutical and medical technology companies address the challenges presented by the country’s aging population and healthcare demands. China’s aging population, which is expected to reach 22% by 2035 and 28% by 2050, presents opportunities for healthcare companies to provide health management solutions for chronic diseases. However, challenges include limited healthcare awareness among patients, geographical gaps in healthcare providers, immense pressures in market access, and difficulties in extending at-home […]

A Comparison of Existing Real World Evidence (RWE) Guidelines
Key Contributors: Neel Patel, Associate Consultant- HEOR, APAC and Rajanikanth Manupati, Associate Consultant- HEOR, APAC In the fast-paced world of healthcare, where the development and approval of new therapies can take over a decade, the need for modern solutions is more pressing than ever. Enter Real World Evidence (RWE) – a game-changing approach that harnesses the power of digitized health data, artificial intelligence, and scalable computational power to revolutionize research and market access. With its potential to offer a robust, cost-effective, and inclusive pathway for better healthcare, RWE is gaining momentum worldwide. Regulatory bodies, such as the European Medicines Agency (EMA), are increasingly embracing RWE, with expectations of wider adoption […]

Navigating the Complexities: Strategies for Asian Pharma Companies Entering the U.S. Market
Key Contributors: Divayum Gupta (Analyst), Tanay Sharma (Consultant), Sowbhagya Suresh (Senior Consultant), Jinsol Kim (Engagement Manager) The United States holds a dominant position in the global pharmaceutical market, accounting for 45% of global sales in 2022. However, Asian pharmaceutical companies face significant challenges when entering the complex U.S. market. The U.S. healthcare system involves multiple stakeholders and a complex multi-payer environment, with regional differences and varying regulations across the 50 states. Additionally, the U.S. market is highly competitive, making it difficult for Asian companies to establish their brand names and compete against multinational corporations. Furthermore, launching in the U.S. market requires substantial investment, and the pricing environment is complex, with […]

Forecasting in the Age of Value-Based Agreements
The pharmaceutical industry faces a host of increasingly complex challenges and critical decisions when attempting to manage and predict their products’ plausible revenue patterns. The mishandling of revenue forecasting and evaluation can result in substantial financial liabilities, which has become more of an issue for manufacturers as products, disease states and additional factors that previously existed in a somewhat predictable space have progressively become more nuanced and idiosyncratic. Over time, the industry has begun to trend toward a more value-based or outcomes-based focus rather than largely centering around creating as much patient access as possible. This creates a more complicated process for manufacturers as there is much more pressure to […]

Key Considerations When Operationalizing Revenue Management
Pharmaceutical manufacturers commonly invest a considerable amount of time, money and additional resources into revenue management. Most would likely say they desire to invest even more because of the significant impact it has on their products’ gross-to-net. But how and where to invest valuable assets is a significant decision. The process of revenue management, including overseeing contracts, adjudication and much more, is highly intricate and requires a great deal of specified knowledge and strategic execution. When a manufacturer is preparing to launch a new product, they may enter into various contracts such as those with group purchasing organizations (GPOs) who negotiate on behalf of healthcare providers to achieve more advantageous […]

Digital Guide to Commercializing Complex Therapeutics
Pharmaceutical manufacturers specializing in Rare Disease, Oncology, Personalized Cancer Immunotherapy, and Cell and Gene therapies encounter a range of complex challenges, including advancements in medicine and technology, changing patient and provider needs, market access, regulatory pathways, pricing transparency, patient reach, patient adherence, and ever evolving disease states. Despite spending over $200 million on product launches, 66% of products fail to meet expectations. Traditional commercialization approaches are no longer effective in these fields. This user-friendly digital guide aims to assist in navigating the challenges associated with commercializing complex therapies. Effortlessly jump from section to section based on your areas of interest. As is the case with all EVERSANA capabilities, the guide […]

Past and Present U.S. Public Health Laws and Regulations, and Their Impact on the Corresponding FDA Regulated Products and Industries
To be regulated by the FDA, foods, cosmetics, human and animal drugs, biologics, tissues, medical devices, combination products, and tobacco products have to meet the federal public health definitions. This white paper provides an overview of past and present U.S. public health laws and regulations and their impact on FDA-regulated products and industries. It highlights the role of the FDA and discusses the historical context behind the enactment of public health laws, such as the 1906 Food and Drugs Act and the 1938 Food, Drug, and Cosmetic Act, as well as subsequent amendments and acts. It emphasizes the importance of ensuring the safety and effectiveness of regulated products through requirements for […]

Pricing Complexities of a Combination Therapy
This article, authored by EVERSANA’s Asia Pacific team, discusses the increasing use of combination therapies in oncology and the challenges associated with pricing them. Combination therapies, which involve a backbone therapy and one or more add-on therapies, offer better clinical outcomes but pose difficulties in determining their value and pricing. One challenge arises from the fact that combination treatments are evaluated as a single therapy, but the individual components are priced independently. The value attributed to the add-on therapy is limited as it is calculated based on the residual value of the combination after removing the price of the backbone therapy. Additionally, combination therapies may not demonstrate cost-effectiveness even if the […]

Integrating the Pillars of Global Pricing Governance
How Emerging Trends are Demanding a More Holistic View to Drive Maximum Value As the complexity of investments needed to secure patient access to therapy increases, so does the impact of these investments on a company’s net revenue. Each decision needs scrutiny, not only on its own merits and how it can affect patient access and affordability, but also to understand how one price change or contract proposal can have a cascading effect on the network that ultimately determines a company’s gross-to-net exposure. Furthermore, once a decision is made, ongoing diligence is required to ensure the price is accurately reflected in the market and that any contract terms associated with […]

Seven Challenges Traditional Omnichannel Tactics Cannot Overcome
How pharma companies utilize omnichannel is a significant factor in their brand’s success, especially as traditional efforts continue becoming more and more obsolete and rejected by patients and providers. Many manufacturers are facing pivotal decisions about what direction to go in with their omnichannel efforts, and whether to embrace new innovative techniques. In this article, we dissect seven challenges facing omnichannel in today’s marketplace and how manufacturers can address these obstacles by incorporating strategies that drive brand adoption by meeting patients’ and providers’ needs, easing processes, creating functional interconnectivity and more. Learn more about how manufacturers can approach omnichannel in a more effective fashion that produces positive results for patients, […]

The Future of Omnichannel is Here. Don’t Get Left Behind.
The standard for omnichannel continues to evolve whether pharma companies like it or not. A next-gen approach to this crucial component of the patient journey is essential to maximize success and best optimize valuable assets. In this article, learn how redefining omnichannel efforts does not have to require investing additional resources. Instead, companies can adapt their current investments to incorporate a more robust and fully integrated omnichannel model that conclusively overcomes access, affordability and adherence barriers. Discover how next-gen omnichannel can heighten brands’ success by modernizing outdated techniques and processes, and how EVERSANA’s model increases therapy adoption by patients and providers by prioritizing their needs and preferences, resulting in greater […]

Create a Comprehensive Experience by Optimizing Forward-thinking Omnichannel Strategies
Deploying successful omnichannel strategies requires manufacturers to evaluate their tactics and ensure they are meeting patients’ and providers’ needs in a modern and effective way. In this article, we lay out a check list of criteria for manufacturers to incorporate into their omnichannel strategy for heightened results that will elevate their brand among competitors, best serve patients and providers, and deliver a strong ROI. Learn what tactics manufacturers should include in their omnichannel strategy check list for more effective and profitable results.

Role of Decentralized Trials for Newer Drug Approvals
Decentralized clinical trials (DCTs) are an innovative approach to conducting clinical research where aspects of the trial are carried out remotely, allowing patients to participate from their homes or local healthcare facilities. DCTs offer several advantages over traditional trials, including increased patient participation and diversity, improved convenience and flexibility for patients, and faster, more efficient trial execution. They have been particularly valuable during the COVID-19 pandemic by enabling the continuation of clinical research while minimizing the risk of exposure to the virus. Health authorities, such as the FDA and EMA, generally support the use of DCTs but emphasize the importance of addressing regulatory compliance and potential challenges associated with remote […]
Navigating an Ever-evolving Canadian Market Access and Reimbursement Environment
These are just some of the trends currently shaping the Canadian market access environment. Achieving favourable market access and reimbursement outcomes for new treatments in this ever-changing landscape requires intimate knowledge of the Canadian landscape, processes and key stakeholders, careful strategic planning, sound tactics and skilled execution. Some key steps and considerations to maximize the chances of timely reimbursement are as follows: Up to 18 months pre-NOC: Information-gathering and early strategy work Conduct a market access landscape assessment Identify unmet needs and potential place in therapy Perform a strategic review of the clinical evidence to identify gaps that may pose a risk during HTA review, and potential mitigation strategies Evaluate […]

2023 Outlook Of China Healthcare Industry: Remaining Pragmatic While Moving Up The Value Chain
As we enter 2023, China surprised the world with a swift and decisive shift in COVID policy, demonstrating the country’s ability to be flexible and pragmatic in its decision-making. In contrast to Russia’s persistent adherence to ideologies, China demonstrated its willingness to pivot quickly when necessary. The 14th Five-Year Plan at the 20th National Congress last November emphasized words like “innovation,” “science and technology,” and “international,” setting the foundation for a long-term scheme to construct a new global order. China’s ambition to lead this new order by dominating critical sciences and technologies sectors, rather than serving as outsourced labor for foreign companies, marks a shift towards targeting a higher-value position […]
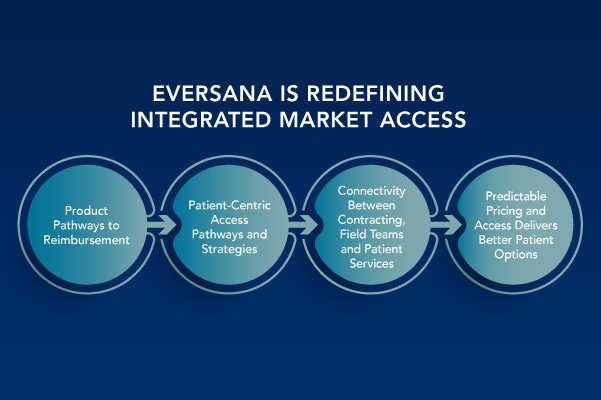
Employ an Integrated Market Access Strategy to Deliver Brand Success
The pharma/life sciences pipeline is vibrant, with ongoing innovation bringing new hope to patients and providers across many therapeutic areas. Against the backdrop of rapid scientific advancement in and precision medicine and targeted therapies in primary and specialty care as well as rare and orphan diseases, today’s medicines continue to create immense complexity for all stakeholders — patients, providers and payers. It has become widely recognized that a diverse array of market access considerations and patient support services are needed to ensure optimal clinical and financial success for any medication. There has been continuous evolution and innovation in the types of programs, services and effective processes that are put in […]

What EVERSANA’s Growing Abstract Count at ISPOR U.S. Means to the HEOR Industry
EVERSANA’s Value & Evidence (V&E) and Data & Analytics (D&A) team collectively had an impressive 27 abstracts accepted to ISPOR U.S. 2023, showcasing EVERSANA’s ability to cross-solve for clients’ HEOR needs. EVERSANA’s Value & Evidence and Data & Analytics teams have had 27 abstracts accepted to ISPOR U.S. 2023, the leading global conference for health economics and outcomes research (HEOR). Chris Cameron, PhD, EVERSANA’s Chief Scientific Officer, and others will be on-site and are looking forward to connecting. Learn more about the event and schedule a meeting with EVERSANA today! Chris Cameron, PhD, Chief Scientific Officer, EVERSANA How did your team manage to produce such a high volume of abstracts […]

Use of Machine Learning to Accurately Size Market Potential and Optimize Sales and Marketing Resources
Machine Learning as a strategic lever in healthcare. Recent advances in Machine Learning (ML) now make it possible for pharmaceutical companies to more accurately identify patient populations, including misdiagnosed patients with rare diseases and other diseases that have a high prevalence of symptomatic related diagnoses, along with their healthcare providers. With this knowledge, it also becomes possible to more accurately allocate sales and marketing resources, which is critical to the successful launch of new therapies and the treatment of patients. In our latest article, read how EVERSANA takes a multi-pronged approach that leverages deep clinical knowledge, patient insights, claims patterns and machine learning to help clients size their market potential […]

Case Study: Optimizing use of Field Reimbursement Managers
Navigating the complexities of insurance coverage can be a time consuming and challenging task. Field Reimbursement Managers (FRMs) were introduced as a way to help healthcare providers address issues or challenges related to securing timely access to treatments for patients, and for securing timely and appropriate reimbursement for therapies that the practice must purchase and administer to patients. Given the important nature of this role, it’s critical for manufacturers to understand how well these FRMs are meeting the needs of their customers. In our most recent case study, discover how EVERSANA’s team of Specialty Consultants helped one manufacturer evaluate both their FRMs as well as those of their competitors, supporting […]

Case Study: Waste Reduction through Packaging Optimization
Pharmaceutical companies are being challenged to develop more effective delivery mechanisms of their therapies in order to reduce the amount of product waste. Recent changes in legislation, including the Waste Reduction Act of 2022 and adoption of Section 1847A of the Social Security Act have made it critical for pharmaceutical companies to optimize drug packaging configurations in order to reduce product wastage – or be required to provide rebates with respect to the discarded amounts of said drugs to Medicare. In our latest case study, discover how EVERSANA’s team of Specialty Consultants used a proprietary, multi-variant forecasting model to reduce wastage from 20% to below 10% and significantly reduce wastage […]

Adult Vaccine Landscape in APAC: The Need for Change
Content for this article was contributed by EVERSANA’s Asia Pacific team. Do Adults Need Vaccines? Immunization should not end with adulthood. Using immunization to prevent infection should be a life-long process, as adult vaccination provides benefits at both individual and country levels, reducing the overall burden of preventable diseases. However, in practice, adult vaccinations are underused. Adult vaccines prevent losses in productivity and help maintain socio-economic stability, essential in developing countries. Higher immunization rates through adult vaccination also help to create herd immunity. Adult Immunization: A Neglected Issue in the Asia Pacific Region Immunization policies in APAC are primarily directed at vaccinating infants and children. This focus overlooks the importance […]

Access Challenges for Severe Infectious Diseases
Content for this article was provided by EVERSANA’s Asia Pacific team. The global burden of infectious diseases is increasing, especially for severe diseases such as invasive aspergillosis, a rare infectious disease, which has a 90% mortality rate. These diseases have poor prognoses and high mortality rates. Disease severity and mortality rates have been increased by anti-microbial/antibiotic resistance. Large gaps exist in the pipeline, with only 18% of antibiotic clinical development being innovative. Manufacturers face challenges in addressing the lack of differentiation in their drugs. In addition to clinical unmet needs, different markets offer a variety of access challenges. Complete the form below to learn more about US, EU5 and APAC […]

Emerging Trends in the APAC MedTech Market
Content for this article was contributed by EVERSANA’s Asia Pacific team. APAC’s MedTech market will soon be the second largest in the world. This growth presents both opportunities and challenges across the region. Booming APAC MedTech Market: The best time to enter is now Over 1.3 billion people will enter their sixties by 2050 in the Asia-Pacific Region. The current supply of MedTech products is far from adequate to meet the resulting demand for high-tech diagnostic equipment, implants, therapeutic appliances, and consumables. The APAC medical devices market alone is already worth $105 billion and is estimated to grow at a CAGR of 6.8% to $150 billion by 2027. Global MedTech […]

Challenges (and Solutions) to Rapid Omnichannel Transformation
Omnichannel can offer countless benefits and notably improve provider and patient journeys. But current marketplace challenges are disrupting success with omnichannel efforts for some manufacturers, and hindering their quality of service to healthcare professionals and the patients they serve. In this panel discussion from the 2022 Digital Health Coalition and moderated by Aaron Uydess, EVERSANA INTOUCH Executive Vice President, Customer Experience and Analytics, three industry experts discuss the challenges facing omnichannel, best practices to overcome those challenge and more.

Evolution of Digital Apps to Total Wellness Solutions
Content for this article was contributed by EVERSANA’s Asia Pacific consulting practice. There is a remorseless demographic logic to increasing health costs. Life expectancy increases, the population ages, and “lifestyle” diseases penetrate further and earlier. How can public health stakeholders resist the trend? One intervention point is promoting the benefits of healthy, active lifestyles. Government-backed awareness campaigns and initiatives to improve healthy lifestyles have been tried with varying degrees of success. Digital applications can provide additional momentum, offering easily accessible support to everyone travelling towards a healthy lifestyle. The health and wellness industry is experiencing exponential growth, as shown by the rapid increase in the number of healthcare-focused tech startups. […]

Benchmarking Your Specialty Brand’s Rx Pull-Through Against Competitors In and Out of Your Network
As an executive at a specialty drug manufacturer, you know having access to your brand’s pull-through key performance indicators (KPIs) inside your network is only a very small part of a much bigger picture. In order to know what good really looks like, you need to know two things: How your brand is performing when physicians send prescriptions (Rxs) to specialty pharmacies outside your network How your competitors are performing both inside and outside of your network for the exact same KPIs Unfortunately, visibility into this type of detailed comparative data for specialty brands is limited by two major factors – the absence of data for your brand outside of […]

The Evolution of HTA and Its Impact on Drug Prices in Japan
Health Technology Assessment Status in Japan Health technology assessments (HTAs) are increasingly used worldwide to assess the clinical and economic impact of drug treatments and technologies, inform health policy, and guide drug pricing and reimbursement. In Japan, HTA occurs after the debut of a product and when companies enter the system, whereas in the UK HTA is scheduled to begin before the launch of the product. The purpose of using HTA in Japan is to adjust a portion of the price premium and complement current pricing rules. The reimbursement price to be applied nationally in Japan is determined by the Ministry of Health, Labor, and Welfare (MHLW) and approved by […]

How Specialty Pharmacies Can Elevate Your Channel and Distribution Strategy for HCP-administered Buy & Bill Products
A successful channel and distribution strategy for Buy & Bill (B&B) products should be centered around the partners you sell to and through. That means considering any third-party logistics (3PL) providers, specialty distributors (SD) and specialty pharmacies (SP) who will touch your product between the manufacturing/packaging site and patient administration. Taking a holistic view of the entire product and patient journey will give you the insight you need to make thoughtful and cost-effective decisions as you pull together this critical part of your overall strategy. Procurement and Administration Options for HCP-administered B&B Products With the continuing rise of HCP-administered therapies for multiple disease states in the last five years, it […]

Plasma derived medicinal products – What is the way forward?
Content for this article was contributed by the EVERSANA Asia Pacific team. Plasma-derived products have a long history of providing benefits, especially in fractions addressing coagulation. Plasma is a rich biological substance and one of the main components of blood, constituting about 55% of total blood volume. The worldwide plasma protein market is expected to reach ~$29B by 2023, with North America constituting 45% of the market by value, followed by Europe and Asia Pacific with 27% and 20% respectively. The therapeutic functions of plasma-derived medicinal products (PDMPs) are diverse. It serves as a raw material for manufacturing several plasma proteins used to treat a wide range of rare diseases […]

Single-Cell Multiomics: Opportunities, Challenges, and the Evolving Business Model
Content for this article was contributed by EVERSANA’s Asia Pacific team. Single Cell Multiomics is a Fast-Growing Market Space with a Wide Range of Applications Single cell multiomics is a new and exciting space in the life sciences industry and research community. Advances in single-cell isolation and barcoding technologies have provided opportunities to profile DNA, mRNA, and proteins at the single-cell level. Multiomics analyses, such as multidimensional genomics, proteomics, transcriptomics, and metabolomics have been of great value in understanding cellular events. The technology has been applied to areas of human biology such as oncology, cell biology, neurology, stem cell, and immunology. The technology holds enormous potential for decoding virus biology […]

Successful Early-Stage Out-Licensing Requirements
The attention on vaccines during the COVID-19 crisis highlighted the role of biotech companies and positive sentiment increased for the sector. In 2021, a record number of biotech (100+) companies went public and listed companies soared to an all-time high. However, today investors have reset their expectations for the sector considering the current economic trends. The S&P ETF for biotech companies has plunged to half of its all-time high, and the impact has also been felt in private markets. Although life sciences investors remain cash-rich, closing investment rounds at 2021’s valuations is no longer a possibility. As valuations reduce, biotechs are postponing their public offerings. Only nine biotechs went public […]
EVERSANA’s Value & Evidence Team’s Work Tops List of Most Read Articles in 2021 in Journal of Comparative Effectiveness Research
If you work in the field of Health Economics and Outcome Research (HEOR) in the life sciences industry, you know just how valuable the Journal of Comparative Effectiveness Research (JCER) is. The publication features peer-reviewed studies from HEOR teams across the globe and is the go-to for researchers and scientists to stay on top of articles ranging from disease conditions to best practices. The journal just celebrated 10 years as a subscription-based publication this past year, and in the listing of Top 2021 articles by readership, the top two were led by researchers from EVERSANA’s Global Value & Evidence team. The top-read article was a Preliminary Communications article titled “Matching-adjusted […]

Case Study: Leveraging Machine Learning Models in Patient Identification and Behavior Prediction for Rare Disease Indications
When a mid-sized pharmaceutical company was challenged with trial and adoption of their immune thrombocytopenia (ITP) drug due to fierce competition and the lingering impact of COVID-19, they turned to the team of data and analytics experts at EVERSANA to help them overcome this challenge. In this latest case study, see how the use of machine learning and predictive modeling helped this client identify distinct patient personas, targeting these patients and their HCPs who had a high probability of trialing their drug. As a result, they were able to maximize their sales and marketing impact, driving 80% of their new patients over the course of 9 months. Download the case […]
Reimagining the Patient Care Ecosystem
Improving Access, Removing Barriers and Speeding Up Time to Therapy Patient demand for telehealth and a flood of industry challenges are disrupting the traditional healthcare landscape. Lack of patient awareness, access to care and overburdened clinicians have created significant wait times to see a provider and a prolonged journey to diagnosis and treatment. The lengthy patient journey coupled with the absence of transparent financial processes has increased the need to innovate the care journey. But what if delivery of care was reimagined, making care more accessible and providing greater transparency throughout the process? In this webinar, industry experts Dan Snyder, Chief Marketing Officer at UpScriptHealth; Hiten Chawla, Vice President, Marketing […]
Customer Centric Approach to Medical Affairs Leveraging Digital Technology
As technology advances the needs of healthcare professionals (HCPs) and patients evolve. The onset of the COVID-19 pandemic accelerated digital adoption among these groups. Medical Affairs departments now require a digital strategy in addition to medical strategies, including technology and innovation roadmaps to offer omnichannel solutions to ensure communications are occurring via the customer’s preferred channel. Concurrently, the complexity of treatment options and algorithms is driving the need for easy access to the latest data and information to ensure the safe and effective use of products and therapies. This is especially relevant in oncology, hematology, immunology and rare diseases. Embracing a customer centric approach and leveraging digital technology can be […]
EVERSANA’s Continued Commitment to Rare Disease
World Orphan Drug Congress USA 2022 As a continued commitment to the rare disease space, EVERSANA was a proud sponsor and participant of this year’s World Orphan Drug Congress USA (WODC). Leaders including Maria Kirsch, General Manager, Patient Services, Simon Andrews, Senior Vice President, Head of Analytics, and Scott Snyder, Chief Digital Officer attended and spoke at sessions on topics including eliminating affordability and adherence barriers of rare disease patients, data and analytics, artificial intelligence (AI) and patient finding. In addition, members of the EVERSANA team had the pleasure of networking and meeting many foundations who share a mutual vision of creating better patient experiences and bringing effective therapies to […]

Is Telemedicine Here to Stay?
Prior to the COVID-19 pandemic, healthcare delivery had been predominantly face-to-face. COVID-19 exposed the capacity limits of the healthcare system, and forced the system to optimize its methods of healthcare delivery by considering non-conventional methods such as telemedicine. Download our article to learn how our telemedicine matrix can inform companies’ mid-to-long term brand strategies.

Established Pharma Brands – A Good Investment?
Offering high cash flows with low clinical and commercial risks, established brands are gaining attention. Big pharma, such as Merck, GlaxoSmithKline (GSK), Pfizer and Sanofi, are spinning off their established brand drugs into new companies. Merck announced that it would be creating a new company, to house its women’s health, well-known established brands and biosimilar businesses, representing around $6.5 billion of Merck’s 2020 revenue. Pfizer and GSK combined their consumer-health businesses of established brands and spun off the entity. Others, including private equity (PE) firms, are also building portfolios of established brands. Established brands provide trusted, high-quality products that have passed clinical and commercial hurdles and accrued brand equity. These […]

An Innovative Approach to Comprehensive Key Opinion Leader (KOL) Mapping
Traditional key opinion leader (KOL) mapping techniques are heuristic and often haphazard: EVERSANA™ has developed an innovative and robust approach that dramatically improves results. We base our ability to identify and prioritize KOLs across disease areas on new technologies and datasets that have made it possible for us to customize searches for many client objectives, problem statements, geographies and target therapy areas. The results have been significant gains in accuracy, comprehensiveness and efficiency, producing a superior result more swiftly compared to using traditional brute force methods.

Evolution of Integrated Mental Health Treatment Models in Southeast Asia (SEA)
Implement Mental Health Integrated Care Models with A Trusted Partner Integrated-care models for mental health conditions implemented in China and Japan have demonstrated the efficacy and cost-effectiveness of such models. SEA is the next frontier of implementing these models and stakeholders in SEA countries have begun assessing and implementing such models. However, infrastructure, numbers of trained personnel and hospital budget issues must be resolved before these models can be successfully implemented in these markets. Pharma companies should be proactive in engaging government agencies and payers to resolve some of these infrastructure and budget issues. Complete the form below and download our article to learn more about the care models that […]

Challenges of Scaling Up Cell Therapy to New Geographies
Cell therapy has emerged as one of the most promising disruptive innovations in the pharmaceutical industry. However, despite its commercial promise, companies developing cell therapies have not been able to take full advantage of its massive potential. Cell therapies offer a massive commercial opportunity In 2021, the cell and gene therapy (CGT) market was valued at $5.2B and is expected to increase at over 30% per year, reaching $25B by 2027. Globally, there are 56 cell therapies approved for clinical use. CGT products account for ~12% of the pharma industry’s clinical pipeline and ~16% of its preclinical pipeline. Further, there are 816 cell therapies in development accounting for 22% of […]

Booming but Constrained: Digital Therapeutics for Mental Health in APAC
Digital therapeutics (DTx) are a fast-emerging class of therapies that use software to treat disease, both as standalone treatments and for treatment optimization. Applications range from improving patient adherence to supporting physicians in managing patients remotely. DTx has been much hyped as transformational across the healthcare ecosystem by bringing personalized medicine to all through AI and real-world data at lower costs. Additionally, the growing aging population and rising healthcare costs are driving the demand for digitalization, from diagnostics and monitoring to therapy, as digital therapies help reduce costs and improve treatment outcomes. In Asia, problems arising from poor mental health are major contributors to years lost due to disability. Over […]

Need for RWE in Consumer Health and Its Impact in Managing Lifecycle of Consumer Health Products
Healthcare has never been more important to the world than in the past two years and it will remain central during the economic recovery. The pandemic’s impact on medical and healthcare systems has emphasized the value of real-world evidence (RWE). This value is now becoming apparent in consumer health, as well as its traditional applications in prescription (Rx) medicines. Using real-world data (RWD) and RWE to support decision-making is not new. It is increasingly used to support regulatory approvals and market access decisions for prescription (Rx) medicines. RWE has formed the basis of safety profile evaluation, risk management, and ongoing benefit-risk assessment for decades. RWE is used during Rx product […]

Is Your Digital Communication Strategy Up to Par?
In today’s competitive global market, pharmaceutical companies cannot afford to waste time or resources on strategies that do not fit patient needs or generate scripts. Interconnectivity between all stakeholders and a 360° view of everything that touches the patient are essential when mapping each patient’s and HCP’s unique and differentiated journey. But how do you ensure you are making the right connections and ensuring all key stakeholders have access to tailored education around treatments? In this webinar, brought to you by EVERSANA™, digital communication and design experts will dive into the history of digital communication in healthcare — and how digital channels are changing how pharma interacts with stakeholders. This […]

Case Study: Defining the Rare Disease Patient Journey to Support Commercialization of Therapy
Rare diseases are complex, and with limited research available, therapeutic options are often limited. Gene therapy, a growing area of clinical research, is showing great promise in treatment that may be life-altering for patients with many rare diseases. A client specializing in gene therapy for a particular rare disease came to EVERSANA looking to better understand the patient journey identify and target their therapy to patients and healthcare providers. While frequently diagnosed at birth, symptoms and comorbidities of this disease can change over time, making identification of suitable patients challenging. The client had limited data available from primary research and clinical trials, but was lacking in real-world data that examined […]

Demystifying Data and Analytics – How to Advance Clinical and Business Objectives
The need to develop a strong, comprehensive data-analytics strategy to support pharma and healthcare initiatives cannot be stated strongly enough. Data is unbiased; it can validate hypotheses or provide direction. But the primary goal is to use data analytics and modeling to develop a more accurate view of what’s really going on in the lives of real patients being treated with complex prescribed treatment regimens, and to generate data-driven insights that inform interactions with patients, prescribers, payers and channel partners. In today’s competitive global market, manufacturers can’t afford to waste time or resources on strategies that don’t generate patient access and prescriptions or address patient and prescriber needs. By carrying […]

FDA Moves Cybersecurity Into the Product Life Cycle
Due to rising cyber-attacks and the potential to cause harm to patients, medical facilities and hospitals, the U.S. Food and Drug Administration (FDA) has recently increased scrutiny of cyber controls in FDA premarket submissions of medical devices. Manufacturers must prove that devices, including software-as-a-medical device (SaMD), do not present cybersecurity vulnerabilities that may affect the device’s safety, effectiveness or security. The FDA recently summarized the significance of the situation, stating, “Cybersecurity incidents have rendered medical devices and hospital networks inoperable, disrupting the delivery of patient care across healthcare facilities in the US and globally.” Both Congress and the FDA recently introduced actions addressing the problem. The bipartisan PATCH Act (Protecting […]

Impact of Patient Reported Outcome Measures on HTA Decisions for Rare Diseases
As rare diseases become an increasing area of global focus, pharmaceutical manufacturers are taking a closer look at how patient-reported outcomes (PROs) may be used to improve uptake in HTA evaluations. Although “rare” suggests not many people are affected with a condition, in the EU between 6,000 and 8,000 different rare diseases affect an estimated 30 million people collectively, while in United States over 7,000 rare diseases affect more than 30 million people Use of patient-reported outcomes (PRO) in rare disease research and clinical practice offers the potential to improve patient care and outcomes and may also help in critical decision-making, especially for orphan medicines. In our latest white paper, […]

EVERSANA Featured in NIH Study Detailing the Cost of Treating Rare Disease
“The IDeaS initiative: pilot study to assess the impact of rare diseases on patients and healthcare systems” EVERSANA™ was the only data and analytic organization invited by a consortium of provider organizations and the NIH to participate in a study with the National Institutes of Health’s National Center for Advancing Translational Sciences (NCATS). The study, “The IDeaS initiative: pilot study to assess the impact of rare diseases on patients and healthcare systems,” was published in the Orphanet Journal of Rare Diseases. The study provides new evidence of the potential impact of rare diseases on public health, including the number of individuals with rare diseases and their medical costs being on par […]

What You Need to Know as the European Union Embarks on Joint Health Technology Assessment (HTA)
Facing the “Fourth Hurdle” Member states of the European Union benefit from a centralised marketing authorisation process for medicinal products. Also, since its implementation in 1993, the European Medicines Agency (EMA) has assured pharmaceutical companies the right to commercialise products that underwent a centralised marketing authorisation in the second largest (and most diverse) single pharma market. However, it remains “the responsibilities of the Member States [to define] their health policy and for the organisation and delivery of health services and medical care. The responsibilities of the Member States shall include the management of health services and medical care and the allocation of the resources assigned to them” (Art. 168, “Treaty […]

How Do We Pay for a Cure? How to Put a Price on Life-changing Treatments
The global cell and gene therapy market is expected to reach $13.8 billion by 2026, expanding at a compound annual growth rate of 12.4%. — Global Cell & Gene Therapy Manufacturing Services Market by Type – Forecast to 2026 Report With over 2,000 ongoing clinical trials in regenerative medicine, patients across the world will soon have even greater access to a large number of life-changing therapies for a variety of diseases. In its 2022 state of the industry report, the Alliance for Regenerative Medicine (ARM) expects at least seven cell and gene therapy approvals in Europe by 2023. A handful of curative therapies have already begun to come to market, […]
Gene Therapy in Today’s Digital World
Rare diseases are an emerging global public health priority, with a staggering impact on the more than 300 million people worldwide who are living with them. There are more than 6,000 rare diseases, 72% of which are genetic, and 70% of those genetic rare diseases start in childhood. Gene therapy holds promise for treatment, and researchers in over 2,000 clinical trials worldwide are studying how and when gene therapy can treat rare diseases. Social media and the internet have been a conduit to finding others living with the same disease around the world, providing a sense of community to the significantly smaller patient population. Of online interactions, 93% start with a search […]

How Can Utilizing Digital Therapeutics Provide Better Care to the Veteran Population?
EVERSANA’s Joe Perekupka, Chief Commercial Officer, participated in a keynote panel discussion at the recent DTx West Summit: “How Can Utilizing Digital Therapeutics Provide Better Care to the Veteran Population?” Joe was joined by moderator Debra Reisenthell, Founding CEO, Freespira; and panelists Ryan Sadlo, VP, Growth, Wellsheet; and Ruth Lowenthal, VP, Total Rewards, Xcel Energy. Providing care for veterans is no longer just about contracting with the VA. Many employers have taken measures to provide better care to their veteran employees. The panel explored how to address the veteran population with digital therapeutics, whether through a large employer or by partnering directly with the VA. Check out the recording to […]

Understanding Current Trends and Needs: Medical Information Contact Center Services
“Understanding Current Trends and Needs: Medical Information Contact Center Services,” a poster by EVERSANA’s Michael DeLuca, Senior Vice President, Medical Affairs, and Keyur Brahmbhatt, Director, Medical Affairs, along with Evelyn Hermes-DeSantis, Director, Research and Publications, phactMI, explores the significant role Medical Information (MI) plays in addressing inquiries from healthcare professionals, payers and patients and ensuring pharmaceutical products’ safe and effective use. In addition, this succinct piece assesses results of a survey conducted by EVERSANA, in collaboration with phactMI™, to define consideration criteria when selecting a global MI Contact Center service provider and identify major reasons for switching providers. Download the poster now or schedule a meeting with EVERSANA’s MI experts.

Succeed When Others Fail: Avoiding 20% in Investment Waste During Launch
It’s a long-standing perception that pharma overspends during launch, but we did not have the data or methodology to understand or validate this notion. Until now. New Research by the Numbers 10: Recent analysis across 10 traditional launches where the manufacturer opted to commercially launch a new drug $345M: The total cost for a manufacturer to commercially launch a new drug under traditional means is estimated at $345.6 million. $267M: An alternative outsourced commercial launch model averaged $267.4 million in investment. 23%: Overinvestment — confirmed Closer look: Results are consistent across company size and therapeutic expertise. The research team quantified the cost of a traditional launch by analyzing real-world examples. […]

Importance of Impactful Medical Information Content
Medical Information (MI) services have evolved as technology has advanced and healthcare professionals’ and patients’ needs and preferences have changed. Understanding the audience, identifying the most beneficial content format, and utilizing the preferred channels of communication are crucial for an impactful medical strategy. Pharmaceutical companies must have technology and innovation roadmaps for omnichannel solutions to ensure communications occur effectively through the patients’ and providers’ preferred channels and to structure content in a user-friendly, easy-to-navigate and digestible format. Industry trends suggest the following considerations when developing MI content: Leveraging component-based authoring tools and looking for ways to leverage content across multiple channels, including online self-service medical portals and chatbots, which leverage […]

How to Ensure Speed and Efficiency When Launching in Europe
Today is an extraordinary time for medical innovation. The pharmaceutical industry is yielding protection against diseases, focusing more on rare and complex disease treatments, and the world is getting better health outcomes. Global life expectancy is more than 70% higher today than it was in 1960 as diseases that were once considered terminal are now treatable. In 2020, the European Union (EU) made a commitment to continue progressing into the future of healthcare with the Pharmaceutical Strategy for Europe. This plan aims to evolve regulatory framework that supports industry and promotes research and technologies that will reach patients’ therapeutic needs while addressing market failures. Specifically, the Strategy will focus on […]

A Holistic Launch in Europe Is Possible, But Change Is Necessary
Unless you live in Europe or have commercialized a product in this region, you may not realize that the European pharmaceutical market is a huge contributor to global health, and it is currently undergoing market-wide changes. Here are a few key facts to know about Europe’s pharmaceutical markets: Europe’s population is more than double that of the U.S. More than 400 million people live in the European Union (EU), and almost 750 million people live in Europe. The EU is changing the market’s infrastructure to support innovation and address “market failures,” such as unmet needs in networks of regulators, health technology assessment (HTA) bodies and payers. The Pharmaceutical Strategy for […]

How to Eliminate Access, Affordability and Adherence Barriers in 2022
Today, everyone expects both digital and human outreach, and manufacturers face the challenge of trying to strike the right balance between tech and in-person touchpoints to enhance patient and healthcare provider (HCP) engagement. Despite all the clear benefits of digital interactions, 40% of customers prefer speaking to a real person on the phone; and for more complex issues, 80% of customers want to speak to a live service agent. Manufacturers must understand exactly how patients and providers are consuming information, connecting with others and completing daily tasks in order to solve for access, affordability and adherence challenges. It’s time to adopt a new equation that combines the forces of high-tech and […]

QUIZ: How Mature Are Your HCP & Patient Engagement Strategies?
In the increasingly virtual world we live in, there’s no denying the instrumental role that technology plays in engaging patients and healthcare providers (HCPs). With heavier reliance across industries on text alerts, automated phone reminders and digital ads that bring product promotion directly to consumers, people expect this same level of digital outreach for their medications; but that doesn’t mean in-person touchpoints should disappear. How are you leveraging technology in your patient and HCP outreach strategies? Like many of us, you might have relied on digital outreach during the pandemic, and now you’re exploring new ways to better integrate in-person and digital engagement strategies. Not sure if or where your […]

A YEAR OF ACTION: Why Data-Backed, Integrated Commercialization Strategies Are Must-haves in 2022
By applying transformative commercialization models, nurturing digital transformation and trailblazing in global expansion, we can get therapies to patients around the world who are still waiting for treatment options. For the past two years, the pharmaceutical industry has proven that it can adapt to change. In 2020, pharma pivoted to manage the coronavirus pandemic, and in 2021 the industry was met with the resounding need to keep pushing, keep evolving – so it did. While some change was overdue, such as streamlining outdated processes with leading technologies, other changes were radical and transformative. In the past two years, changing market conditions have forced pharma to consider new strategies, resulting in […]

Client Story: An Ode to EVERSANA
At EVERSANA, putting our clients and patients first is at the forefront of everything we do. Recently, when EVERSANA ENGAGE’s Bausch & Lomb (B&L) team closed out their last event of their speaker series, they didn’t just receive any feedback. They received a poem. Jodi Ceberio, Managing Director, Client Services, said that this poem is a special thank you to Alex Marquez, who leads the EVERSANA ENGAGE Meeting Services Team. “Alex has been instrumental in growing our B&L business and we are so grateful for her!” “I want to send my sincere thank you for all you have done this year to help us,” said Dr. Jim Hoffman, B&L representative and featured poet. An […]

Pharma Europe 2021 : Panel
A Data and Digital Enabled GTM Strategy for Long-term Business Impact Rohit Sood, Executive Vice President, COMPLETE Commercialization, joined a panel of industry experts to discuss why an aligned and cross-functional go-to-market strategy has never been more important and how to balance strategic priorities in this Pharma 2021 conversation. Watch the recording: Meet with our experts.

Bio-Europe “Collaboration Close-Up”: Exploring the EVERSANA – Shorla Pharma Partnership
Launching in today’s unpredictable market is a journey with new challenges around every corner. While the oncology pipeline is rapidly growing, stringent competition and an evolving provider environment are putting intense pressure on manufacturers to build effective commercialization infrastructures and launch products at unprecedented speeds, which comes at a steep price. In this session, Shorla Pharma, a specialty pharmaceutical company, shares their partnership journey with EVERSANA to bypass the traditional commercialization model that’s too inefficient, costly and cumbersome. Mike and Sharon share their vision of an integrated model that’s streamlined to maximize brand value and improve patient access and outcomes. Panelists include Sharon Cunningham, CEO, Shorla Pharma and Mike Ryan, Executive […]

The Need for RWE Studies in APAC and the Impact on Product Life Cycle Management
Advances in technology and globalization have enabled significant growth in the pharmaceutical industry over the last decade. Digital initiatives and focusing on patients have also conditioned researchers to think beyond traditional data collection to support product value propositions. As a result, real-world evidence (RWE) is gaining in importance and becoming an important part of managing the product life cycle, not least in Asia-Pacific (APAC). Home to over half the world’s population, APAC is a vast commercial opportunity for pharmaceutical and healthcare companies. Many APAC markets are still emerging and promising rapid market growth, while Japan’s and China’s market sizes make them priorities for pharma. Markets such as Singapore, Australia, Korea, […]

Webinar: Coupon & Co-Pay Conference 2021
Leveraging eServices and Technology to Improve Access and Co-pay Card Utilization Payers and self-insured employers are driving enrollment in high-deductible health plans, shifting financial risk to patients and increasing out-of-pocket costs. As a guest presenter at Informa’s annual Coupon and Co-Pay 2021, EVERSANA’s Tom Doyle outlined how manufacturers can leverage predictive analytics, omnichannel and personalized engagements to ensure patient affordability and access. Watch now: Go Beyond Co-pay to Deliver Customized Patient Support and Drive Therapy Adoption Payers and self-insured employers are driving enrollment in high-deductible health plans, shifting financial risk to patients and significantly increasing patient out-of-pocket costs. As a result, patients are at risk of a gap in medication adherence, discontinued use […]

Health Technology Assessment in the Asia Pacific Region
Health technology assessment (HTA) is the systematic evaluation of the properties and effects of a health technology, addressing the direct and intended effects of the technology as well as its indirect and unintended consequences. In many countries, it is a primary evaluation tool for making pricing and access decisions, providing a platform for understanding economic trade-offs. HTA varies considerably across APAC and depends on country-specific factors, such as the proportion of public investment in health, political support, access to quality health information and technology infrastructure. With the rising cost of healthcare, HTA can be a useful tool to inform decision-making about universal healthcare (UHC ) and promote an equitable, efficient […]

EPP Studio Live Webinar — Impact of COVID-19 Pandemic on German Healthcare System
Leading up to the recent German elections, the topic of healthcare took center stage for the aging German population. With nearly 90% of the population participating in Statutory Health Insurance (SHI), increased financial pressures have widened the gap between SHI coverage and anticipated expenditures. During this EPP Studio Live Webinar, Douglas Foerster, Senior Vice President of Pricing & Market Access for EVERSANA, and Mathias Flume, Head of Drug Development for KVWL, discuss how various political parties are responding to these challenges and the potential impact on market access and pricing in Germany. WATCH NOW:

Webinar: Discover Patient Support Program Needs for Patients with Rare Diseases
In a world where patients face more medication barriers than ever, patient support programs for rare disease patients are critical in providing value-based care that yields a palpable and lasting impact. Manufacturers are acutely aware that product access and affordability is key to improving patient adherence and are more than willing to allocate the time and resources to achieve better results. Yet despite significant investments in technology, dedicated care teams and educational materials, manufacturers’ patient support programs are facing a decline in prescription fill rates and conversion to therapy rates. By leveraging deep insights into access, affordability and adherence barriers – and how corrective actions improve adherence – manufacturers can […]

EPP Studio Live Webinar — EU Joint Procurement of COVID-19 Vaccines: A Model for Future Market Access in Europe?
The COVID-19 pandemic has changed the way we live and the way we respond to global healthcare crises. During this EPP Studio Live webinar, Douglas Foerster, Senior Vice President of Pricing & Market Access for EVERSANA, shares a brief historical summary of how the European Union negotiated the acquisition of COVID-19 vaccines through an unprecedented, and somewhat controversial, joint procurement process. Mr. Foerster and Professor York Zöllner of the Hamburg University of Applied Sciences discuss the pros and cons of this joint procurement approach as they explore various scenarios under which this process may offer future market access considerations of cost, speed, efficiency and equity. WATCH NOW:

A Data-Driven Commercialization Pathway to Expedite Rare Disease Diagnosis and Adherence
“We often think about big data as this big, cold, robotic thing; and I think it reveals, in real time to us daily, that there are opportunities for many small, important moments and many impactful, emotional things to happen for patients and caregivers.” —Amy Hutnik, General Manager, Agency, Advisory & Evidence Services When the life sciences industry thinks of data, it’s often associated with clinical development and regulatory decision-making; but the power of real-world data (RWD) and real-world evidence (RWE) also plays a key role in commercialization strategies. The critical mass of industry data continues to grow every day. With a constant input of data into commercial strategies, manufacturers must […]

Building a Life Sciences Digital Ecosystem
In the life sciences world, we hear more and more of the requirement for digital ecosystems in various therapies. What do we mean by these, and what benefits do they provide? A digital ecosystem is a network of multiple stakeholders who collaborate to enhance delivery to patients. A healthy, sustainable ecosystem benefits patients, prepares companies to adapt to economic changes, builds patient loyalty, creates new revenue streams and lowers patient acquisition cost. Creating a sustainable digital ecosystem implies creating sustainable elements. Examples outside the life sciences industry include the following: BMW leverages its first-in-class data management, allowing its users to have premium security and privacy. Google Nest is collaborating and […]

Assessing Market Opportunity and Entry Challenges in Mental Health Disorders
Although mental health disorders affect almost 264 million people worldwide, they are often misunderstood and misdiagnosed, and access is poor. The World Health Organization (WHO) reports that 80% of people in low- and middle-income countries receive no treatment for their mental health disorders. As well as being underdiagnosed, mental health disorders are also poorly studied and an extremely challenging target for pharma companies. Obstacles include the high cost of conducting trials and generic competition. An Asian pharma company recently partnered with a small biotech firm to develop and commercialize a novel therapy for indications within depression and anxiety. The company needed to characterize the nature and scale of the opportunity […]

Increasing the Value of Community Health Workers
In developing countries, frontline community health workers (CHWs) extend basic healthcare services to communities. These extensive CHW networks provide services that improve various health indicators. The Philippines has set a goal of one CHW (“Barangay Health Worker”) in every village, while India is close to meeting its goal of one CHW (“ASHA”) per 1,000 people countrywide. CHWs assist health authorities in raising awareness and implementing public health initiatives in rural and distant regions, as well as in encouraging the adoption of healthy behaviors. There is often great scope to improve the value offered by CHWs by providing them with additional or superior products and services – but making it happen […]

Getting Treatments to Patients When They Need Them Most: Next Gen Commercialization in Oncology
Commercializing oncology products is extremely complex, but successfully launching new therapies to market is essential for patients and providers in the fight against cancer. In 2020, about 10 million people died from cancer, proving even in a global pandemic that cancer continues to be a leading cause of death globally. While the oncology pipeline is packed with innovative, new treatment options, pharma companies need to ensure their commercialization model is evolving as well; otherwise, these treatments may never reach the patients who are waiting. Not only is the oncology pipeline thriving, 90% of the companies delivering novel cancer treatments are emerging biopharma. Launching in this niche market is challenging for larger, […]

Novel Antibiotic Opportunity in China
China, with its large population and extensive healthcare system, is the second-highest user of antibiotics in the world. Such widespread use has inevitably led to high levels of antibiotic resistance in the population. When isolated, it becomes clear that a significant majority of the infecting bacteria are gram-negative. Within these bacteria, antibiotic resistance has grown significantly to the point of becoming a serious health threat. China has responded to this increasing antibiotic resistance with preventive measures, such as setting up a surveillance network, prescription control and placing restrictions on formularies. In this restrictive environment, we reviewed the Chinese antibiotic market to gain insight into the potential market access for innovative […]

Assessing the Feasibility of ITCs
When looking at different healthcare interventions for a disease, there may be limited direct evidence on how the treatments compare with each other in terms of their efficacy, tolerability or another measure of interest. In this video, EVERSANA’s Chris Drudge, MPH, PhD, Associate Project Manager – Value and Evidence, shares how indirect treatment comparisons (ITCs) can be used to fill in the blanks and compare treatments that have not yet been directly compared in a randomized clinical trial. However, ITC feasibility assessments (FAs) are also necessary to understand whether ITC is suitable and, if so, which ITC method(s) are most appropriate. Watch the full video here: Assessing the Feasibility of […]
Simplifying EU Distribution to Maximize Cost Efficiency and Speed to Market for Patients and Manufacturers
COVID-19 ignited a spark of innovation in the healthcare industry, forcing global markets to reconsider drug development and commercialization processes. The European Union (EU), specifically, is taking carefully planned steps into a new phase of pharma with recent changes, including the Pharmaceutical Strategy for Europe. But one element of the European pharma industry that remains constant is product distribution through parallel trade. In 2012, parallel trade activity rose by up to 25% in some countries; but distributing a product across territories with diverse regulations, cultures and languages is complex. Now, there is an even more efficient way for manufacturers to distribute treatments in Europe. The answer is one end-to-end commercialization […]

Digital Therapeutics: Where Technology Meets Healthcare
Digital health is growing rapidly across the world, supported by technologies that not only assist disease monitoring or prevention but can also reduce the healthcare burden. Digital technologies have improved equity of access and enabled European patients to monitor and self-manage their health. It has improved efficiency, effectiveness and quality of patient care while also reducing costs. Wider adoption will shape a predictive, preventative, personalized and participatory healthcare ecosystem. One arm of digital health is digital therapeutics (DTx), which are evidence-based therapeutic interventions driven by software programs. DTx are considered part of the solution for the rise in preventable chronic diseases and rising healthcare burdens. It is supported by the […]

How Manufacturers Can Optimize and Organize Support Services for Specialty Drugs Through a Patient Touchpoint Analysis
Currently representing almost half of the overall pharmacy benefit spend, specialty drugs are classified as being high-complexity, high-touch and high-cost medications. As healthcare providers (HCPs) and patients turn to these more expensive products to treat rare, complex and/or chronic medical conditions, the need for increased patient education, adherence and support services has been elevated. To best understand the patient experience and their needs, healthcare systems are turning to patient journey mapping. Using this tool, healthcare entities such as the provider’s practice, the insurer, the specialty pharmacy and manufacturers are developing and providing their versions of patient support services. Click here to download the white paper.

Adapting Patient Access Programs to Asia
In Western markets, pharmaceutical companies have developed many approaches to patient access programs (PAPs), normally emphasizing initiatives that, while helping patients, also support pricing and reimbursement initiatives, such as: Early access programs before market authorization. Financial access initiatives before achieving reimbursement. Value-based contracts, managed-entry agreements or outcomes-based programs for achieving reimbursement. Non-financial assistance post-reimbursement. The need for PAPs is higher among Asian patients, and many countries have accessibility complications — transport, communication, access to healthcare — as well as affordability issues. In Asia, most PAPs target financial access with simpler schemes, such as volume-based discounts, per-patient price caps, price bundles and income-based discounts. These financial access measures are especially important […]

Optimizing Your Digital Marketing Spend and Field Deployment to Impact Script Adoption
In today’s competitive global market, pharmaceutical companies can’t afford to waste time or resources on strategies that don’t generate scripts or fit product and patient needs. Without data-informed, fully integrated campaigns, there’s a missed opportunity to mine deep insights that can inform next steps and accurately pinpoint efforts to doctors and patients who could benefit from additional touchpoints during the campaign, yielding higher results. Rather than guessing which HCP targets most disproportionately impact script fills, tracking provider networks through data and analytics can provide discrete insight for creating more effective, targeted provider outreach strategies. When data is actively and regularly informing campaign design, execution and interconnectivity, insights and messaging become […]

Competitive Benchmarking In Trade – Answering The Who, What, Why and When
Last month our colleague Derek Cothran addressed the importance of using secondary research to benchmark your Patient Support Program (PSP) against obvious and not-so-obvious competitors. We’d like to continue a discussion around competitive benchmarking in this month’s blog, but this time focused on how it can be used to optimize a channel or distribution strategy for both established and start-up manufacturers. Specifically, we’ll discuss the benefits of considering options that perhaps have not been done before, why taking risks can be more beneficial than you might think, why it’s imperative to know the why behind a competitor’s decisions as well as touch on the optimal times to competitively benchmark your […]

Navigating the Transition to Post-Approval Pharmacovigilance With EVERSANA and ArisGlobal
Partnering with a contract research organization (CRO) provider during clinical development fills an important role in the product life cycle, providing pharmaceutical companies with pharmacovigilance expertise and support during clinical trials. After product approvals, it can be tempting for companies to remain with their CRO partner; however, the commercial phase of the product life cycle has more mature pharmacovigilance needs that must be met. For example, unlike post-market safety, pharmacovigilance during clinical trials is extremely controlled, with many factors in the pharmaceutical company’s favor, including: A designated treatment population, with specific parameters for inclusion and exclusion for treatment. A focus primarily on reporting serious adverse events (SAEs), which are delivered […]

Case Study: Launching a New Therapy for HER2-Positive Metastatic Breast Cancer
When MacroGenics partnered with EVERSANA, they had less than five months to launch their first product in the midst of the global pandemic. To meet their timeline and streamlined launch, MacroGenics needed a commercialization partner with an end-to-end platform that would allow them to build their capabilities expeditiously and strategically.

The Patient Access Paradox: How the New CMS Rule Could Prioritize Drug Pricing Before Clinical Decision-Making
In January 2023, co-pay programs will be put to the test, consequently examining how well your brand can adapt to the Final Rule changes to meet patient and provider needs. Our recommendation: Don’t wait – start solutioning a patient-centric approach to access and affordability now.

A SpaceX Philosophy to Launching in Pharma
In thinking about the economics around the launch of pharmaceutical products, it is useful to compare the situation to another area that has seen its economics evolve in recent years: space travel. For decades, the only reusable space vehicle was NASA’s space shuttle. When the space shuttle was in operation, it could launch a payload of 27,500 kilograms for $1.5 billion, or $54,500 per kilogram. Between 1970 and 2000, the cost to launch a kilogram to space was reduced but then remained fairly stagnant, at an average of $18,500 per kilogram. Eventually, the space shuttle program was brought to an end, deemed too expensive, complex and unsafe. Now, Elon Musk’s […]

Considering a Virtual Advisory Board as a Viable, Cost-Effective Option for PMR Planning
Advisory boards are key in helping pharmaceutical manufacturers refine product positioning and guide value messaging throughout the product life cycle. Like many processes this year, advisory boards have been impacted by the pandemic’s travel restrictions and have been unable to convene as traditional in-person meetings, but the need for their thought leadership remains. As manufacturers adapt, leaders might feel limited to multiple one-on-one discussions with board members or be struggling with the number of challenges associated with virtual meetings: Disengaging, disjointed board conversations via video Absence of hands-on tools, such as illustrating concepts on whiteboards Lack of creativity and ingenuity As board meetings continue to be held virtually, EVERSANA is helping our partners leverage this option more efficiently to meet the demands of today’s hybrid world. Read more from our global commercialization experts about how to create engagement and cultivate creativity for your advisory board.

The Rise of Digital Therapeutics Opportunities in China
The expansion of digital health technologies is growing exponentially and has increased through the pandemic. One focus for investment for this therapeutic area is China, where digital growth has outpaced most of the western world in areas such as e-commerce. While such growth is exciting, business models that offer returns are less understood. EVERSANA’s global commercialization experts explain how we’re examining the Chinese market to help our partners better understand China’s digital health evolution. DOWNLOAD NOW Schedule a meeting with one of our experts in the Asia Pacific region today!

Transform Your Medical Information Contact Center Into a Strategic Asset for Customer Engagement
The pharmaceutical industry is constantly evolving, and manufacturers are re‑strategizing, embracing technology and innovation, to meet the needs of the providers and patients they serve. Successful product launches on a global scale are critical for manufacturers to reach more patients and providers. As a result, manufacturers are streamlining operations, relying on fewer vendors and service providers to improve process efficiency; reduce cost; and ensure outreach synergies, alignment and consistent messaging. As commercial launch strategies evolve, manufacturers must also re-envision their medical plans, including their medical information strategies. It’s time to move beyond the traditional “call center” model and into a multichannel contact center focused on building a center of excellence […]

PharmaVOICE Webinar: Next Gen Commercialization Model for Oncology
In a new 60-minute virtual panel, “Next Gen Commercial Models in Oncology,” PharmaVOICE Editor Taren Grom sat down with industry leaders to discuss how changing market dynamics and a rich pipeline in oncology are creating a need for next gen commercial models.
Ask the Expert: One-on-One with Amy Hutnik
The pharma industry’s needs have evolved beyond one-dimensional playbooks, disconnected promotional efforts and limited ability to assess stakeholder engagement. Instead, manufacturers need to adopt a comprehensive omnichannel model that allows for data-driven planning and real-time analysis of results from marketing campaigns, field activities and patient services programs to create a cohesive brand experience with maximum impact. EVERSANA commercialization expert Amy Hutnik answers an important question every manufacturer needs to know about omnichannel: How does a manufacturer’s commercialization model affect the success of their omnichannel strategy? Additional Reading Download our latest infographic for an in-depth look EVERSANA’s omnichannel activation model.

Pharma USA Webinars: Personalize HCP Experiences for Deeper Engagement
EVERSANA was proud to participate in two Pharma USA 2021 sessions. This webinar, featuring EVERSANA’s Amy Hutnik, General Manager, Agency, Advisory and Evidence Services, covers the following topics: Harnessing AI to inform your sales approach and meet your customers’ need for both human interaction and digital engagement. Pull don’t push: Build more meaningful relationships with customers by engaging only when necessary and delivering value-add supporting content. Supplement face- to-face interactions with a digital backdrop of integrated channels for an enriched “surround-sound” customer experience. Personalize HCP Experiences for Deeper Engagement Pharma USA 2021 is a business-critical virtual experience for pharma leaders and health innovators. This cross-functional gathering aims to build partnerships, […]
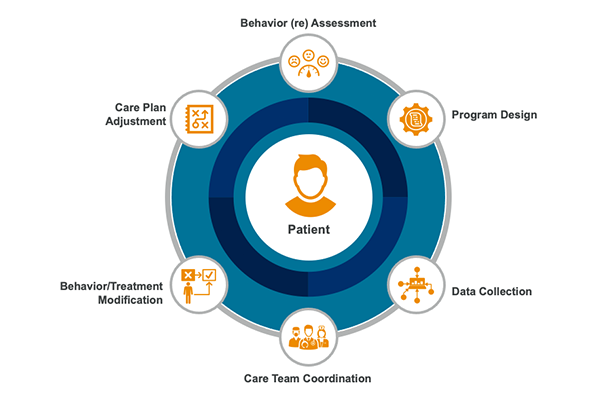
Engaging Oncology Patients and Providers in The Age of COVID And Beyond
The engagement model for oncology patients and providers is failing, and challenges brought on by the COVID-19 pandemic have only accelerated the trend. Not only are pharmaceutical reps restricted in the types of information they are allowed to communicate, but their access to providers in oncology practices is also more limited than ever as a result of COVID and productivity concerns.

Are You Benchmarking Your PSP Against Others? If So, Who and What Are You Comparing It Against?
Many of our clients are looking for ways to ensure their Patient Support Program (PSP) is not only serving their patient and provider populations well but that it is doing so in a fiscally responsible way. Additionally, they are seeking validation that their PSP is at least at parity or superior to the competitor products’ PSPs. To assist these clients, we propose benchmarking their planned or existing PSP against what is already on the market. In this entry, I’d like to discuss how secondary research can be one of the most efficient and cost-effective ways to gather industry insights that allow you to benchmark your PSP against competitors. I’ll also […]
Informing NAMs Payer Strategies and Improving Contracts With Integrated Commercial Services
Pharmaceutical manufacturers rely on national account managers (NAMs) to build relationships, negotiate contracts and get their products in front of payers and pharmacy benefit managers who will clear the way for patient treatment coverage. Challenges Facing NAMs Typically, NAMs rely on the customer or the health plan to direct contracting strategies and product expectations, and they often manage contracts in a silo without input from other commercial services teams. While NAMs may have some data insights, the disconnect between commercial services causes them to inefficiently leverage data or miss insights that could lead to optimized contracting strategies. Without data integration, NAMs cannot pinpoint the patient populations and treatment indicators for […]
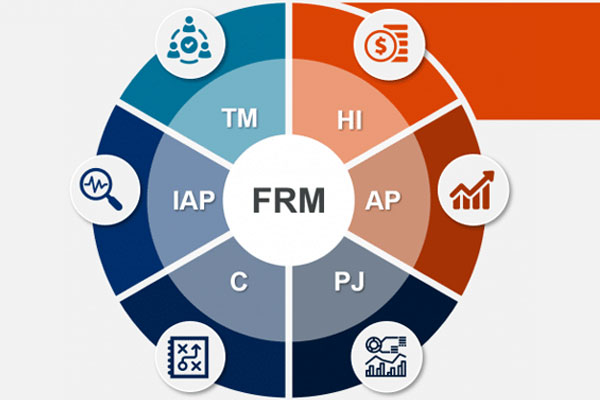
Revolutionizing FRMs With Data Connectivity and Commercial Services Integration
FRMs, sometimes referred to as field reimbursement specialists or patient experience specialists, are the key drivers in patient pre-authorization and billing and coding processes, and play a vital role in the prescription and adoption of specialty drugs. While field reps focus on selling, FRMs focus solely on supporting healthcare provider (HCP) offices and helping office staff get past complex processes and procedures that can hinder speed-to-therapy for patients. Challenges Facing FRMs The highly technical nature of the FRM role is already challenging and becomes further complicated by a lack of connectivity and process inefficiency with hub and data and analytics teams. Manufacturers and life sciences companies realize that connectivity for […]

3 Ways Field Teams Can Shape the Market Before and After Product Launch
“It is not how much you have or know, or even who you know; it is how well you adapt to the inevitable changes along the way. As Heraclitus, the Greek philosopher, said, ‘Change is the only constant in life.’” – Marc P. Bernarducci, Senior Vice President of Field Solutions – Clinical Field Medical (FM) teams have witnessed a few of the pharmaceutical industry’s biggest changes. From the PhRMA Code on Interactions with Health Care Professionals to the swift movement of the Digital Age, FM has always found a way to adapt – and these teams must continue to do so. Today more than ever, market shaping is becoming vital […]

The Secret’s Out: The Impact of Net Price Transparency
Pricing system pressures are increasing for the top four markets in Europe. Price transparency, changes to public health plans and curative therapies are set to disrupt traditional drug pricing. To navigate this ever-shifting landscape, companies must carefully consider product adoption and launch sequencing, and understand the potential impact that net price transparency might have. In this webinar, EVERSANA’s Alan Crowther, General Manager, Global Pricing and Market Access Solutions, and Ed Corbett, Senior Principal, EVERSANA™ CONSULTING, EMEA, discuss: Situation: What’s the current global landscape of net price transparency? Implications: How are the changes going to impact current in-market products and future product launches? Actions: What actions do firms need to take […]

Audit From Anywhere: Upgrade Your Quality System Audit Programs
As the world strives every day to adapt to changes this year, one change that pharma manufacturers are adapting to is the impact of COVID-19 on product safety audits. From supplier audits to evaluating internal or affiliated sites with the FDA, travel restrictions have made it difficult to access and be on site for audits at facilities as has been done in the past. We have learned, however, that remote and cross-functional collaboration can be successful to solve the industry’s leading challenges, and quality system audit programs are not an exception. Check out this article by Steve Beauchamp, Director, Quality, for tips on making your audit program a benefit to your organization, even in a time of global transition.

Are You Tracking the Performance and Satisfaction Ratings of Your Patient Services?
The types of services patients need for support change and fluctuate over time, particularly as a product moves through its lifecycle. The patient services necessary to drive access, affordability and adherence to your product in the launch phase can be markedly different from the patient services that accomplish the same goals at later stages of your product’s lifecycle. This makes it critical that manufacturers continually check to ensure they are still providing relevant and differentiating services to all stakeholders throughout all stages – from diagnosis to therapy initiation and adherence. It should also be noted that providing the right services at the right time is not enough. You also need […]

Visualizing EVERSANA’s Omnichannel Activation Model
The pharma industry’s needs have evolved beyond one-dimensional playbooks, disconnected promotional efforts and limited ability to assess stakeholder engagement. Manufacturers need to adopt an omnichannel model that goes beyond “marketing” to provide actionable insights that better inform commercial strategies and elevate brand success. Expect more from your investments in datasets, technology and promotional campaigns; expect more from your omnichannel strategy. Download the full visual for an in-depth look into how EVERSANA’s omnichannel activation model works.

Webinar: Under Pressure: Global Trends in Net Price
In the past few years, net price transparency has become a subject of debate in the international pharma community. As pricing reforms and shifting pressures on pricing systems continue to intensify, how will they impact markets in Europe and Asia-Pacific? Watch our webinar featuring EVERSANA’s Alan Crowther and Amardeep Udeshi for a discussion (presented at World EPA Congress 2021) on pricing trends in Europe and Asia-Pacific, risks and opportunities, and the next steps pharma needs to take. Specifically, this webinar looks at the following: Trends in Europe Net Price Transparency in the EU Trends in Asia-Pacific View the full webinar: To learn how these trends will impact your new product launch strategy Talk […]

Turning Theory Into Action: How Real-World Evidence Drives Clinical Research and Improves Patient Outcomes
Life sciences companies have made significant investments in real-world data (RWD), but most established providers can deliver only a fraction of what’s needed to be impactful. Breaking down traditional healthcare silos for a more innovative approach to drug development can shorten the research and development timeline while substantially reducing wasted investments along the way. From the beginnings of drug discovery through post-market safety monitoring, partnering with pioneers who can deliver best-in-class RWD and RWE becomes critical in getting better, safer therapies to patients faster. Read Brigham Hyde’s latest article to learn more. Learn more about how we’re helping our clients make RWD a core part of their clinical and medical […]

How to Scale Your Learning & Development Department: Ask The Expert
For years, Learning and Development teams have been asked to “do more with less.” When budgets tightened, teams would get more creative allocating their resources and time. But today – in the midst of changing market dynamics and a global pandemic – teams have been asked to “do more for more.” They are finding themselves preparing content and training internal clients across various departments – not just the commercial team. As manufacturers navigate growing business needs, how can manufacturers ensure their sales teams continue to perform at a maximum level? EVERSANA’s Denise Fullowan, Vice President of Learning and Development and Cari DuBose, Manager of Sales Training, explain why many manufacturers […]

Webinar: CMS Final Rule on Cost-Sharing Assistance
The CMS final rule on drug reimbursement addresses how co-pay coupons and vouchers are being exempted from deductibles by PBMs. The rule seems to ensure that the full value of manufacturers’ coupon and voucher programs accrue to the enrolled patient, meaning that the portion of the cost of the drug paid by the manufacturer program counts toward a patient’s deductible and not be exempted from it, as happens when a PBM (or payer) implements an accumulator program. While the intent of the final rule seems to help the patient, CMS makes the manufacturer responsible for determining whether a PBM has implemented an accumulator program, which is difficult (if not impossible) […]

Change Is Happening in the EU: What Pharma Companies Need to Know About the European Union’s New Pharmaceutical Strategy
For the first time, the European Union is undergoing a major overhaul of its pharmaceutical industry with the new Pharmaceutical Strategy for Europe, which was launched in late 2020. Through this strategy, the European Union (EU) is making changes to its infrastructure with the goal of building a holistic, patient-centered, forward-looking pharmaceutical landscape for all EU member countries and patients. Focusing on patient access, treatment affordability and sustainable innovation, the European Commission outlines four key areas in which they will take legislative and non-legislative actions to move this progressive, patient-centric plan forward: Delivering for patients: Fulfilling unmet medical needs and ensuring accessibility and affordability of medicines Supporting a competitive and innovative European pharmaceutical industry Enhancing resilience: A diversified and secure supply chain, environmentally sustainable pharmaceuticals, crisis preparedness and response mechanisms Ensuring a strong […]

Watch Now: PharmaVOICE Panel on Next Gen Patient Services Models
The Future of Data-Driven Patient Support Is Now The use of health data technologies and analytics in the life sciences industry continues to evolve, but many manufacturers are still crawling around in the dark. With limited in-person patient and provider-rep interactions, hubs that lack synergized technology and data are struggling to understand access and adherence barriers – and, most importantly, new and unexpected patient needs. Despite best efforts, prescription rates continue to drop as newly launched products experience increased patient drop-off; and companies are struggling to support patient acquisition, retention and conversion. In a new 60-minute virtual panel, PharmaVOICE Co-Founder and Editor Taren Grom interviews EVERSANA’s Maria Kirsch and Kevin […]

Leveraging RWE to Enhance Experiences Across the Patient Journey
In a tech-driven healthcare ecosystem in which patients are engaged consumers, product value is contingent on treatment outcomes and patient success. With digital tools and resources at their fingertips, today’s patient is empowered to make informed treatment decisions; and physicians need enhanced support to ensure they’re meeting patient needs. Providing physicians with the tools to practice precise, individualized patient care requires manufacturers to go beyond traditional outreach strategies and adopt advanced technologies using real-world data (RWD). With RWD, companies have the opportunity to produce sophisticated real-world evidence (RWE) that fills in the gaps of each patient’s story. Companies must start by applying RWD and RWE insights when it matters the […]
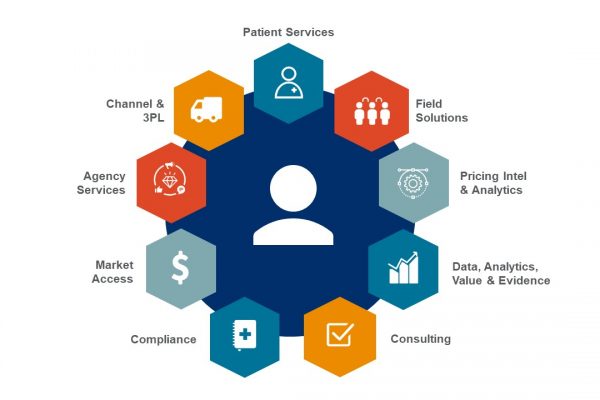
Reinventing Launch: The Gold Standard Of Drug Commercialization
In a world that is rapidly changing, we must evolve beyond traditional strategies to create true impact for patients. EVERSANA’s complete end-to-end commercialization model enables manufacturers to bring their drug to market at a fraction of the cost of “going it alone” or partnering with another pharmaceutical company.

The Crush: How Covid-19 Is Impacting Mature Brand Revenue & Long-Term Value
The impact of COVID is reverberating across all aspects of society and business. In the healthcare industry, hundreds of thousands of patients are not getting proper access to and utilization of therapies that can improve their clinical outcomes. The clinical consequences of this will lead to compromised patient outcomes and further healthcare cost increases. This also leads to negative strategic and financial ramifications for biopharma manufacturers who produce these therapies. Billions of dollars of revenue and tens of billions of dollars of market valuation have been erased over the last year due to COVID-related market conditions, for every dollar of revenue lost can lead to a disproportionate loss in market […]

How to Determine Price and Global Launch Sequence in a Post-COVID World
The globe is facing a multitude of governing and legislative changes that will directly affect pharma and pricing in coming years, with many of these changes initiated by the pandemic. As countries become increasingly interconnected, decisions in one pharmaceutical market will have ripple effects globally.

Transforming Pharma With Industry-Leading Partnerships
EVERSANA is filling an influx of essential commercialization needs to overcome strained resources and support COVID-19 treatment launches. From groundbreaking treatments to long-overdue therapy improvements, there will be at least five new treatments headed to market this year. These innovative partnerships will allow us to stand alongside pharma companies leading the industry in improved patient treatments and outcomes. Read more about the partnerships that are paving the way for cutting-edge therapies this year. Driving Groundbreaking Therapies to Market Partnering with Iterum to support the upcoming launch of oral sulopenem for the treatment of uncomplicated urinary tract infections (uUTIs). Supporting lenzilumab™ with Humanigen to treat hospitalized and hypoxic patients, pending Emergency Use Authorization approval. Leading the commercialization effort […]

Upcoming PharmaVOICE Panel – Next Gen Patient Services Models
With increasingly less face-to-face patient interaction, manufacturers need to rethink traditional solutions in order to alleviate access barriers. Despite best efforts, prescription rates continue to drop as newly launched products experience increased patient drop-off; and companies are struggling to support patient acquisition, retention and conversion. In a new 60-minute virtual panel, PharmaVOICE Co-Founder and Editor Taren Grom will interview EVERSANA’s Maria Kirsch and other industry leaders to discuss how changing market dynamics are creating a need for next gen patient services. During this event, you’ll learn how to: Leverage innovative technology, such as advanced analytics and machine learning, to overcome access barriers; Personalize patient and HCP engagement; and Maximize outcomes […]

A Paul Simms Favorite: EVERSANA makes the list for 2021 Predictions
“Imagine there was a third way … an equivalent to the Apple App Store in our industry … maybe that model could start working in pharma; and, in fact, it is.” For pharmaceutical influencer Paul Simms, commercialization options for biotech and pharma companies aren’t great — in fact, they’re slowing the market down. In 2021, Simms predicts a new option will entice manufacturers and cause a shift in companies that rely on traditional commercial options. In this interview for Impatient Health, Simms advocates for a commercialization option that shares profits and risk with manufacturers, like Uber for drivers and the Apple App Store for app developers. And, he says, this […]

Transforming Commercialization With Industry-Leading Partnerships
Healthcare innovation is being intensified as the industry rushes to find solutions to the pandemic, while furthering research in rare and complex diseases. As pharma companies reignite clinical development, streamlining commercialization is crucial in bridging the gap between treatment manufacturing and patient access. Recognizing this industry need, our team is actively partnering with companies leading the fight against COVID-19 to get life-saving products to market. We’re filling essential commercialization needs to overcome strained resources and support COVID-19 treatment launches at an influx, with at least four groundbreaking therapies headed to market this year. Launch speed and product delivery are being put to the test, and our team of commercialization experts […]
The 10-Year Test: Is It Possible to Plan Launch During Clinical Development?
Time and strategy are keys to launching in an overwhelmed, unpredictable market. Pharmaceutical companies in mid-development of a drug need to look ahead at their launch strategy options and consider what market conditions could look like by the time they’re ready to commercialize their product. By evaluating the value of an asset early in development, pharma companies can gain a better understanding of a product’s value and risk. With assessment insights, manufacturers can make informed clinical and commercial decisions moving forward. Taking advantage of far-off timelines will allow pharma companies to strategically plan where and how to spend critical investments. Read this case study to see how EVERSANA™ helped one APAC-based company assess its product’s market value and plan for a launch 10 years in the future – because it’s never too early to start planning.

Moving Forward in Pharma: Reigniting Revenue for Mature Brands
The world will be moving forward with immeasurable effects from the COVID-19 pandemic, as will the pharmaceutical industry. Fully understanding the impact that the pandemic will have on pharma will take years; however, there are future indications emerging for mature brands. Mature brands, or non-promoted in-line brands, are the bread-and-butter products for pharma companies in a normal market. These brands have historically provided reliable revenue support, allowing manufacturers to research and develop drugs in new treatment areas. Now, approximately $31.4B of these “recurring” revenue stream brands are facing failure due to COVID-19-related impacts. As COVID-19 cuts into pharma’s bottom line, manufacturers need to begin strategizing how they can address the […]

How Better Alignment Propels Your Brand’s Success
As pharma companies race to keep up with today’s market, your brand’s team can’t afford to be out of sync. Yet inefficiencies and miscommunications are common throughout the commercialization process — wasting valuable resources, creating confusion among key stakeholders and slowing progress toward your brand goals. For many brand teams, these problems are often triggered by a lack of alignment between key functions (such as market access, patient and consumer marketing, training, clinical, manufacturing, sales and IT) and their respective industry partners. When members of your brand team and their vendors aren’t aligned, communication gaps can occur, causing confusion and duplicative work efforts. Even worse, these gaps can lead to poor investments in disjointed strategies that are created […]

Emerging 2021 Life Science Priorities
From commercializing high-science therapies to empowering a data-driven patient experience (in the midst of a global pandemic!), we’ve learned that the life sciences industry requires agility, speed and scale. How will 2020 experiences set the emerging trends of 2021? Read below to learn more: Predictive Analytics The implementation of data and analytics has presented many challenges to the industry, from wasted investments in disjointed data sources to staffing gaps. This presents an opportunity, however, for the industry to shift toward integrating data and services into a streamlined solution, aiming to drive decision-making and improve patient outcomes with evidence-based insights. Read: How to activate proactive patient services when you combine the […]

EVERSANA is Fighting COVID-19 Through a Growing Number of Client Engagements
The new year began with a light of hope ignited by the long-awaited COVID-19 vaccine. However, a vaccine is only the first step in fighting the pandemic. Healthcare professionals and drug manufacturers must continue to apply cutting-edge research and technology to overcome challenges related to vaccines, treatments and patient testing. While the industry’s top minds work to develop essential COVID-19 treatments, our team is actively partnering with manufacturers to quickly and safely get these life-saving products into the hands of physicians and patients. Now, more than ever, time and resources are stretched thin and complex product launches require an agile, integrated commercialization partner. As the leading provider of global services […]

PharmaVOICE Digital Influencers: Brigham Hyde
Data Is Transforming the Landscape In a special feature on Digital Influencers, PharmaVOICE Editor Taren Grom sat down with Brigham Hyde, President, Data & Analytics to discuss how data is transforming the life sciences landscape as we know it. From the rise of personalized medicine and value-based healthcare to evolving commercial strategies, the effective utilization of data is critical to solving for better patient outcomes. Watch the video below to learn more about how we’re leveraging data to drive a better patient journey and trends to look for in the new year. Read Brigham’s Digital Influencer profile below. “Influencers have the power to positively change the status quote because of […]

How to Enhance Provider and Patient Engagement in Tech-Savvy Healthcare
Pharmaceutical representatives have always been a welcomed resource and consistent office visitor for healthcare providers (HCPs). Today, in a world operating largely virtually, pharma reps are re-strategizing HCP engagement in the same way providers are rethinking patient outreach. As telehealth intercepts in-person appointments and office visits with HCPs become limited, providers will have to determine how, where and when they will spend time with sales reps moving forward. Even in a virtual world, providers are still interested in having face-to-face, value-driven conversations with sales reps. Reuters reported that almost 70% of HCPs prefer live remote or phone detailing to webinars, e-meetings, email, website browsing, bots and online journals — yet […]

Leading the Way in Digital Health With Tech-Driven Clinical Pathways
Digital health technologies have become integral to patient care since the beginning of the COVID-19 pandemic and will remain essential healthcare tools. As many patients and healthcare providers are embracing digital health devices, clinical pathway developers have an opportunity to lead the way in digital technology implementation — if they choose to. Healthcare providers rely on clinical pathways to guide patient treatments with diagnostics and medications that have been approved by the Food and Drug Administration (FDA) and are eligible for Centers for Medicare & Medicaid Services (CMS) coverage. While digital health tools have gained popularity for improving digital and financial outcomes, most of these devices are not approved by the FDA and are ineligible for reimbursement, forcing clinical pathway developers to decide whether or not to add […]

Insights From Dreamforce 2020 Panel: End-To-End Patient Engagement
EVERSANA was proud to participate in this year’s brand-new session “End-To-End Patient Engagement: From Clinical Trials Through Patient Support Programs.“ Bhaskar Sambasivan, Chief Strategy Officer and President of Patient Services, provided valuable insight into the emerging industry trends that are driving digital transformation within life sciences. He explained how manufacturers can embrace innovative technology to better serve patients and increase their speed to therapy. “We need to view patients as partners to truly co-create and build support programs that can be tailored to each patient, as opposed to a one-size-fits-all model.” Click the image below to watch the full session. Personalized Patient Solutions and Engagement Improve Adherence Each patient takes […]

Insights From Trade & Channel Strategies Conference 2020
Now more than ever, pharma manufacturers need to break the cycle of supply chain complexity to better connect patients to therapy. On Tuesday, December 1, EVERSANA’s Scot Buchanan moderated the panel “Emerging and Future Trends in Channel Strategy, Distribution and 3PL” with these industry leaders: Rob Osborne, Vice President, Pharma Trade Relations Express Scripts, Accredo, CuraScript SD Liz Minko, U.S. Distribution & Channel Strategy argenx U.S., Inc. Spencer Miller, Director, National Accounts, Trade Bayer U.S. LLC During this 45-minute session, the panelists outlined their strategic imperatives for the upcoming year and described their greatest opportunity to build a patient- and provider-centric distribution and services strategy. Watch the full webinar […]

DSCSA Guidelines – Best Practices for Compliance and Supply Chain Integration
On November 27, 2023, all drug manufacturers must participate in an electronic package-level traceability system, commonly known as the “interoperability” requirement. EVERSANA is equipped to help manufacturers navigate these new guidelines and apply best practices. Let’s work together to make sure that you stay in compliance with the FDA and meet the demands of your customers throughout the supply chain. Click here for a short snippet of the webinar, “Best Practices for Compliance and Supply Chain Integration” Watch the full webinar:

EVERSANA Receives PM360 Innovations Award for Seamlessly Disrupting Commercialization
EVERSANA’s end-to-end commercialization solution is reimagining traditional pharmaceutical commercialization models for the first time by breaking down industry service silos with integrated approaches and advanced technologies. PM360‘s Annual Innovation Issue was established in 2011 to provide pharma with its first-ever guide to the life sciences industry’s latest advancements. In its December 2020 edition, the publication is recognizing EVERSANA COMPLETE COMMERCIALIZATION as an innovative strategy that is pushing pharma forward and achieving improved results for providers and patients. Our COMPLETE COMMERCIALIZATION model now creates a fourth option for drug manufacturers who are ready to bring their product to market. With a full-scale commercial strategy, operational excellence, and a success share delivery platform, we’re mitigating risk for manufacturers while giving them back control of their treatment’s success. Learn how EVERSANA COMPLETE COMMERCIALIZATION is changing the way pharma companies see commercialization and simplifying the pathway […]

Solving Hard Go-To-Market Challenges [Webinar]
How can looking outside the box help identify innovative approaches that achieve market objectives? Today, in a world where pharma is shifting away from primary–care blockbusters and embracing specialty–care products, looking outside the box is essential. Focusing on HCPs during a product launch is no longer the key to commercial success. Instead, pharma companies must adapt quickly, leveraging artificial intelligence and machine learning technologies to acquire insights and investing resources where they’ll make the greatest financial impact. With a new, agile commercial model, pharma companies can shift with changing market dynamics and optimize patient, provider and payer engagement. In this webinar, EVERSANA is joined by industry leaders from Bausch Health, Evoke and Palette Life Sciences to discuss how pharma companies must overcome launch challenges in today’s dynamic commercial landscape. Watch the full Webinar:

Case Study: Early-Stage Asset Valuation
The rewards of developing a new drug can be very attractive but they come with inherent risks. It is important to ensure that investment decisions on clinical development are made after examining the risk profile of the drug which depends on the therapy area and the nature as well as extent of unmet needs. EVERSANA APAC Leadership recently supported a client to help them make a right decision on how to invest and when to license. Our client was developing an early stage asset in an immunology indication. The drug was still in pre-clinical stages and would take about ten years to launch in an already crowded market. Since the […]

Leadership Briefing – The U.S. Pharmaceutical Market Outlook: The Path to Recovery and the New Normal
During a recent webinar panel, EVERSANA and Reuters gathered industry leaders to discuss the tough questions facing pharma, specifically in the wake of a new U.S. presidency. EVERSANA CEO Jim Lang; Joe Jimenez, Ex-Novartis CEO and CEO and Co-founder of Aditum Bio; and Kabir Nath, Senior Managing Director, Global Pharmaceutical Business, Otsuka Pharmaceutical Co., Ltd., shared their perspectives on the need for new commercialization models and mandates to better promote the value we collectively provide as an industry. In this follow-up Q&A, you’ll find expert insight on: Shifting to value-based care and commercialization. The growing need for data-driven drug development. How to introduce more cell/gene therapies at a sustainable cost. […]

Five Biggest Data Challenges for Life Sciences
Data is transforming the competitive landscape in life sciences. From the rise of personalized medicine and value-based healthcare to evolving commercial strategies, the effective utilization of data is critical to solving for better patient outcomes. There are significant challenges, however, including siloed, messy data quality; slow legacy systems delivering fragmented insights; and the inability to transfer data quickly, easily and securely across disparate systems. Brigham Hyde, President, Data & Analytics, sat down with industry leaders at Snowflake and Compile to discuss how EVERSANA is addressing these challenges with ACTICS, our tech-enabled solution built to optimize end-to-end success for life sciences. ACTICS includes applications aimed at the most valuable client use […]

Five Things You Should Do to Ensure Your PSP Is Ready for Launch Before Your Brand Is
Your brand is preparing for launch, and it’s time to develop a strategy for your Patient Services Program (PSP). Even if you already have one or more PSPs in place for your company’s other brands, it is important to make sure you don’t incorporate a one-size-fits-all approach for your PSP. In order to develop a PSP strategy that meets all of the brand, stakeholder and patient needs, there are multiple important considerations you should evaluate. Here are 5 crucial considerations to make when determining the right PSP for your brand, and more importantly, for patients. Step 1: Define your profiles The optimal place to start developing a PSP strategy for […]

Approaching Clinical Pathway Development as an Art
Much like any artform, creating a clinical pathway requires forethought, innovative design and strategic execution in the hands of the physician — except in the art of healthcare, the most important critic is the patient. While physicians try to perfect the art of patient care, the market realities of sky-rocketing costs and fewer resources influence how their practices are managed. Following a fee-for-service care model often dulls the character and priorities of a practice while pushing the needs of patients to the back burner. Instead, developing clinical pathways that align with value-based care allows for consistent, high-quality care that does not impede on the individual physician’s art of healthcare. Implementing […]

Managing Risks to Improve Health Outcomes: How to Move Population Health Forward in an Era of Uncertainty
At the Canadian Association for Population Therapeutics (CAPT) Annual Conference, Sumeet Singh, Senior Director, EVERSANA, delivered a presentation on pharmacoeconomic analysis and outcome-based agreements and what they mean for Canadian private payers. Watch his session below for answers to the following questions: Are pharmacoeconomic analyses relevant for private payers? Should pharmacoeconomic analyses for private payers differ from those for HTA/public payers? Should a societal or health system perspective be considered at all by private payers?

Four Ways Predictive Analytics Can Strengthen Your Commercialization Efforts
Over the past few years, artificial intelligence (AI) and machine learning (ML) have been trending topics in healthcare but are now being propelled to the forefront of clinical decision support and care delivery. Some organizations, however, have been slow to adopt the utilization of predictive analytics for a number of reasons. From wasted investments in disjointed data sources to staffing gaps, the application of predictive analytics can seem daunting and unattainable. In reality, implementing a data integration strategy can break down the traditional healthcare silos that have made it challenging to execute on the insights gleaned from data and analytics and, ultimately, improve patient outcomes. To address these challenges, EVERSANA […]

Optimizing the Performance of a PSP and Improving Outcomes For All Stakeholders
Patient Support Programs (PSP) have been proven to improve clinical and patient outcomes and help manage patient out-of-pocket cost and prescribed use. Particularly for specialty drugs, the services provided under the PSP umbrella not only help remove barriers to patient access, they provide healthcare providers (HCPs) with tools they can use to help patients better manage their disease. The challenge has been with ensuring a PSP is evolving and maturing in a way that aligns with patient and stakeholder needs as well as the product’s lifecycle. Click here to download the white paper.

ODRD Insights: Building an AI-Driven Data Ecosystem to Drive Commercial Activities
The market landscape, especially for rare diseases, is dynamic and presents a unique set of challenges for the life sciences industry. Unlike the case for broader disease states, most rare disease patients are not correctly diagnosed for an average of seven years due to misdiagnoses, patient data sparsity or ambiguous coding. To address these challenges, the industry is increasingly relying on data-driven insights and predictive modeling for improved decision-making. In his insightful presentation at the 2020 Orphan Drug and Rare Disease Congress, Oodaye Shukla, Chief Data and Analytics Officer, outlined how we can leverage artificial intelligence to advance rare disease patient identification and drug commercialization. Complete the form below to […]

The U.S. Pharma Market Outlook: Path to Recovery and the New Normal
With the U.S. presidential election days away and the continued impact of a pandemic, EVERSANA and Reuters gathered industry leaders to discuss what is to come. Watch as the panel discusses the tough questions facing pharma, the need for new commercialization models, and a mandate to better promote the value we collectively provide as an industry. Watch the webinar on demand.

NORD 2020 Insights: Entering a New Era
EVERSANA was a proud sponsor of the 2020 NORD Rare Diseases & Orphan Products Breakthrough Summit to advance the dialogue on ways to improve the lives of over 25 million Americans living with rare diseases. Our mission is to advance the drug development and commercialization of orphan drugs, amplified by today’s need to create better patient experiences and bring effective therapies to patients around the world. Moments that matter in the patient experience Jim Lang, CEO of EVERSANA, hosted a virtual brainstorm on how to transform the patient journey and create moments that matter along the way. Moderated by Faruk Abdullah, Senior Managing Director, and Penny Bemus, SVP of Client […]

MARKET ACCESS AND TREATMENT OUTCOMES: Shifting From the Volume of Data to the Value of Insights
From Volume to Value: “The best prescription is insightful knowledge.” Former Surgeon General of the United States Dr. C. Everett Koop once aphoristically said, “The best prescription is knowledge.” This thought permeates much of what drives market access, but it is missing the essential word of the sentence, and that is “Insightful” knowledge. Dr. Koop practiced medicine during a time when information was limited to individual practices and groups. Today, hospital systems, payers and pharmaceutical firms have access to excessive amounts of data – and it is our task to turn this volume into something accurate and actionable, something of value. Additional challenges coincide with data saturation as well. In this article discusses how today, hospital systems, payers and pharmaceutical firms […]

Population Health Partnerships To Advance Value-Based Care
The business model for health care in the United States is evolving from a volume-driven model to a consumer-centric, value-driven model. As such, there are new competencies required of hospitals and health systems to effectively manage a population’s health across the continuum of care. Many hospitals and health systems will need to partner with other organizations to gain the capabilities and efficiencies required to provide services under new care delivery and payment arrangements. This article discusses the why, what and how of creating and implementing a population health project with partners. Download the FULL article HERE

Mitigating Risk: When A Curveball Threatens Your Product’s Launch
In this 3-minute video, Mike DeLaroche shares a new commercialization model that mitigates risk and combats curveballs that manufacturers cannot predict when launching products.
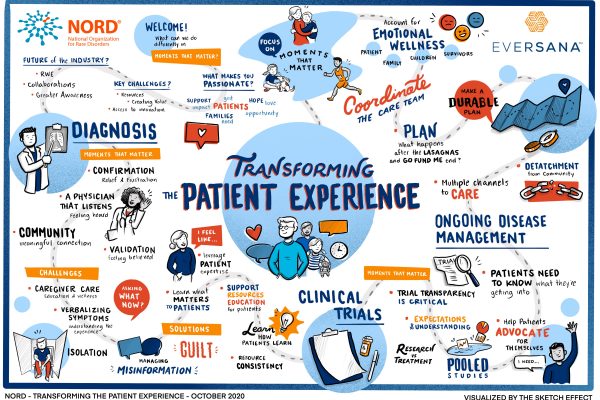
An Illustration: Transforming the Patient Experience
As a fierce ally of the rare disease community, EVERSANA proudly sponsored the 2020 Rare Diseases & Orphan Products Breakthrough Summit. Longtime partners in the advancement of orphan drug commercialization, EVERSANA and the National Organization for Rare Disorders (NORD) are dedicated to improving the lives of over 25 million Americans living with rare diseases. During this event, EVERSANA and NORD teamed up with rare disease experts to brainstorm how to transform the patient journey and create more moments that matter. Hosted by EVERSANA CEO Jim Lang and moderated by Faruk Abdullah, Senior Managing Director, and Penny Bemus, SVP of Client Management, the group identified numerous solutions for treating the patient […]

eBook – 2020 Stories That Shaped Commercialization
While many industries hit the pause button on their operations this year, the pharmaceutical industry never stopped, propelling new innovations to better serve patients, providers and stakeholders. At EVERSANA, we worked closely with manufacturers to tackle uncertain, complex market dynamics and solve pricing, access, reimbursement, adherence, and distribution challenges, and more. As we look forward to 2021, download EVERSANA’s special edition e-Book, “2020: Stories That Shaped Commercialization,” to recap the trends and stories that drove the industry to new heights this year. eBook Preview: Accelerating Patient Outcomes With Data and Analytics The New Gold Standard of Commercialization The Transformation of Digital Therapeutics Strategic Global Product Launches Pivoting the Patient Experience […]

When A Curveball Threatens Your Product’s Launch
Download the article to learn about proven strategies to help shield your company from unwelcome surprises during launch by reducing your financial exposure and creating a more stable and flexible launch operation.

CBI Hub, SPP and eServices Insights: Leveraging Behavioral Health Technology
The Science of Human Resiliency — Behavioral Health Technology to Improve Patient Outcomes and Adherence Over the past several years, pharmaceutical manufacturers have been in pursuit of improving the patient experience by reducing time-to-treatment, developing a more integrated patient view and deriving insights that lead to better care. Additionally, physicians have been seeking new tools that provide deeper insights into their patients’ journeys. Last month at the CBI Hub, SPP and e-Services conference, EVERSANA’s Brian Davis and other industry experts presented a case study to illustrate how personalized messaging via secured, two-way texting improved medication adherence for patients suffering from recurring cystine stones. In this session, the panelists explained how […]

PharmaVOICE Commercial Influencers: Greg Skalicky
In a special focus on Commercial Influencers, PharmaVOICE Editor Taren Grom sat down with EVERSANA’s Chief Revenue Officer Greg Skalicky to discuss approaches to commercial strategies and how disruptive companies are changing the market to better reach and engage patients. The conversation covered commercialization trends, how the industry has changed in the last few years, what is driving innovation within solution providers and small- to mid-size pharmaceutical companies, the role of technology, choosing the right commercialization partner, and the importance of transparency and cultural fit. Click to read the story online

Compliance – The Competitive Differentiator to Commercialization: An Integrated Model Setting the New Global Standard
The Compliance experts at EVERSANA prove how integrated life science compliance has earned its seat at the table by demonstrating the value of increasing data-driven and technology-infused competitiveness in the successful commercialization of a new-age biopharma product.

WODC Insights: Developing HEOR Programs for Orphan Drugs
To successful launch a new orphan drug, demonstration of therapeutic success is crucial for a global HTA submission and securing market access. Manufacturers need to create a compelling value story with real-world data to illustrate how the therapy positively impacts the patient journey. In his informative presentation from the World Orphan Drug Congress 2020, Chris Cameron, PhD, outlined the steps, best practices and timelines for developing a global HEOR program. Click here to watch the full session. Chris also moderated the panel, “Use of Real-World Evidence in HTA Review of Orphan Products” with Trevor Richter of CADTH and Gregory Daniel of Edwards Lifesciences. Click here to watch the full session. […]

NEXT GENERATION COMMERCIALIZATION: A CONVERSATION WITH THE INNOVATORS
Sell, out-license or launch internally: these are the three traditional options to commercialize a pharmaceutical product. Selling and out-licensing are common but cause innovators to lose ownership in an investment that takes years to develop. Launching internally requires an average investment of $125MM+ before the product even hits the shelves. Until now, there was no other way to take a product to market. The newest option – EVERSANA™ COMPLETE COMMERCIALIZATION – affords innovators full access to a complete end-to-end commercialization model. In this panel moderated by EVERSANA’s Greg Skalicky and Jim Lang, EVOKE CEO Dave Gonyer and Zosano CEO Steven Lo explain how selecting EVERSANA as their commercialization partner allows […]

WODC 2020 Insights: Leveraging AI/ML to Advance Rare Diseases
Leveling the playing field The average time to correctly diagnose a patient with a rare disease is 7 years, largely due to data sparsity, misdiagnoses, incorrect prescriptions, etc. Now, through the application of artificial intelligence and machine learning algorithms, we are able to shorten the time to diagnoses of rare diseases and even discover undiagnosed patients on the way. With the recent launch of ACTICS by EVERSANA, our integrated commercial services are further strengthened by best-in-class predictive analytics to analyze data quickly, make predictions and perform smarter, faster actions for life science companies. We shared our expertise at the 10th Annual World Orphan Drug Congress, the premiere meeting on advanced […]

Navigate the Complexity of Proposed CMS Medicaid Rule
In this presentation at MDRP’s virtual summit, Mike Kurland discusses several key regulatory proposals and executive orders and the impact on pharma: Operationalizing Value-Based Contracting – Has the door been opened, and how do we prepare for an increase in value-based strategies? Managing Coupons and Vouchers – Do we have the right data, and what is the impact on best price? Line Extensions – How far does this go, and is your organization set up to assess GP impacts? Medicare – Understand the changes in Parts B and D and prepare for the operational impacts. Executive Order to Buy American – How are you estimating impacts to your VA/Tricare liability? […]

How to Get Your Digital Therapeutic Approved & Reimbursed in Europe and the U.S.
Patients, providers, and payers expect healthcare to be more accessible, intuitive, and adaptable to their needs. Barriers that have held back innovation in Digital Medicine and Telemedicine have been blasted apart with the recent relaxing of CMS and HHS requirements, continuing advances within the FDA’s Digital Health program, and additional reimbursement opportunities emerging globally. Get insights on two critical topics: Challenges and imperatives to obtain approval of your Digital Therapeutic Discuss the challenges and imperatives to obtain approval of digital therapeutics in both Europe and the U.S., the continued advances and changes in digital medicine and telemedicine worldwide, and the roadmap to success. Explore the reimbursement opportunities in Europe and […]

PharmaVOICE100 Recognizes Two EVERSANA Leaders
For the past 15 years, PharmaVOICE has celebrated leaders throughout the life sciences industry who provide inspiration to their peers, colleagues and companies through their innovative and motivational approaches to addressing the industry’s myriad challenges. As defined by PharmaVOICE these individuals: set industry trends and create opportunities out of obstacles; are innovative and have the ability to think outside the box; develop breakthrough strategies, products, and services; are known for pioneering new paths and lifting their companies to new heights; and also take the time to mentor the next generation of industry leaders. Two EVERSANA leaders are recognized in this year’s PharmaVOICE 100: Jim Lang and Peter Marchesini. DRIVING DISRUPTION […]

WODC 2020 Insights: Innovative Value and Access Strategies
Innovative rare disease therapies deserve innovative value and access strategies. With over 1,500 attendees from 50+ countries, the World Orphan Drug Congress featured four days of education, conversation and brainstorming dedicated to drug development and commercialization. EVERSANA’s CEO, Jim Lang, had the esteemed honor of hosting Pfizer’s Vice President of Rare Disease, Patient & Health Impact, Bhash Parasuraman, in a one-on-one keynote panel. Throughout their informative 30-minute discussion, Jim and Bhash emphasized the importance of implementing innovative value and access strategies that ensure more patients receive life-altering therapy. Bhash explained how the industry cannot hold the performance of rare disease therapies to the same standard of care of chronic diseases. […]

The New Gold Standard of Drug Commercialization
Manufacturers spend >$125MM over three years leading up to launch, yet 66% of drugs don’t meet launch expectations. An unpredictable landscape, coupled with inevitable industry pressures, is forcing manufacturers to seek a more complete commercialization approach with less risk and more value.

The Importance of Digital Strategy in Pharma in the APAC Region
The Asia Pacific region continues to show great progress in digital health as pharmaceutical companies look at digital technologies as a tool to drive access and to start developing their digital strategies. To understand the strides and recent evolution of digital in pharma, our APAC team conducted a survey with top pharmaceutical companies in the region. Amardeep Udeshi, Partner at EVERSANA APAC, outlined the results of the survey and provided insightful recommendations during our Digital Symposium on how the industry is changing. Watch Amardeep’s insights and get his take on the survey results

Improving Patient Outcomes Through Digital Health
Digital applications can work cohesively with therapeutics in the treatment of a broad range of diseases, from behavioral health to chronic conditions. In an interview with Debraj Dasgupta, Head of Strategy & Go-to-Market Planning at Nippon Boehringer Ingelheim Co., Ltd., Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, obtained an insightful perspective on digital transformation in healthcare and how pharmaceutical companies in the APAC region and across the world are leveraging digital technologies. Watch the interview in its entirety

The Digitalization of Healthcare in Asia Pacific
The digitalization of healthcare in Asia Pacific is being utilized to enhance the patient-centered approach and expanding access to health. In a candid conversation with Abhishek Shah, CEO at Wellthy Therapeutics, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, uncovered the current healthcare landscape and how digital strategy in pharma is evolving in the APAC region. Watch this insightful conversation:

The Regulatory Landscape in Digital Health
Despite the advances in digital health and digital therapeutics in recent years, there remains an ambiguity in the digital space and uncertainty surrounding regulation and reimbursement of digital technologies applied to pharma, biopharma and medical device companies. In an insightful conversation that was part of EVERSANA’s Digital Symposium, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine, and Martin Culjat, Sr. Vice President, Regulatory Innovation & Digital Medicine, discussed the use of digital technologies in pharma and the regulations surrounding it. Watch the full conversation:

Access to Technology and the Rise of Digital Health in Asia Pacific
The access to technology, development of telehealth and evolution of digital therapeutics are thriving in Asia Pacific; and pharmaceutical companies are levering the momentum to reach to a broader segment of the patient population. Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, interviewed Anish Shindore, Head of Digital Therapeutics at Sanofi, during our Digital Symposium to understand the critical aspects of the use of digital technologies by pharma companies in the Asia Pacific region. Watch the interview now:

Digital Innovation in Asia Pacific
Digital medicine and digital therapeutics are transforming the healthcare industry by changing the care delivery format, and they have the potential to improve patient adherence and outcomes. In our recent Digital Symposium, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, interviewed David Keene, CEO at IntroSpect Digital Therapeutics & Lead, Digital Therapeutics at ATAI Life Sciences, and they looked at the evolution of digital therapeutics and the shift of the use of digital technologies in healthcare. Furthermore, Ed and David highlighted the trends and innovations in digital health happening in Asia Pacific, pointing out the barriers, opportunities and path forward. Watch the interview:

Outlook: Digital & Pharma in Asia Pacific
Asia Pacific is one of the fastest-growing regions for digital innovation in the pharmaceutical and biotechnology industries. Its promising outlook is mainly driven by necessity, commercial flexibility and a broad talent pool. In a candid conversation broadcasted during the EVERSANA’s Digital Symposium, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine, and Ravi Visweswara, Executive Vice President at EVERSANA APAC, reflected on all the changes and trends in the use of digital technology, providing insightful remarks and an outlook into the future of digital in pharma in Asia Pacific. The two industry experts brought a holistic view of digital health in the pharmaceutical sector and highlighted […]

Copay Accumulator & Maximizer Programs Are Changing
A Look at Copay Assistance Programs and Copay Accumulators Copay assistance programs are a known tactic deployed by drug manufacturers to help facilitate access to often expensive specialty medications. These financial assistance programs take a comprehensive approach to supporting a patient’s out-of-pocket costs, inclusive of any applicable deductibles and drug cost-share responsibilities – up to a patient’s annual out-of-pocket maximum. The strategic thinking behind these programs is that without them, prescriptions become unaffordable and lead to product abandonment and/or poor adherence. Basically, copay assistance is paramount in achieving the best patient outcomes. Payers, particularly PBMs, have generally adopted the position that these programs introduce conflict in their ability to manage […]

Insights from ISPOR 2020
Shaping the future of patient care. From a panel discussion to podium and workshop presentations, EVERSANA shared insight at this year’s virtual ISPOR 2020 conference. More than 1,300 attended the conference from 54 countries; and our experts demonstrated the impact of comprehensive HEOR capabilities, including economic modeling, evidence synthesis, value communication and reimbursement strategies, across all stages of the product lifecycle. Below is an overview of our participation, and each links to a downloadable PDF presentation. It represents the breadth of the value we bring in helping shape the future of patient care. Chris Cameron – WORKSHOP: Developing and Implementing an Indirect Treatment Comparison Program to Support Global HTA and […]

Ask the Expert: Digital in Pharma | The Critical Questions
EVERSANA is uniquely positioned to follow the trends and introduce new models and strategies to maximize the value of pharmaceutical products through Digital Medicine Solutions and Execution. Digital Solutions can be deployed to impact the entire life cycle of a product in unprecedented ways. In a “digital tell-all” conversation, Ed Cox addresses four critical questions on pharma’s digital strategy. Schedule a 1-hour FREE workshop with Ed and his team to discuss your digital strategy. [REQUEST A MEETING]

Podcast: The Future of Digital Therapeutics
The Digital Therapeutics market continues to evolve at a rapid pace. In this podcast, hosted by Erica Sosnowski, host of the podcast series “The Platform”, we discuss current trends in the life sciences industry and the future of digital therapeutics with Edward Cox, the Global Head of Digital Medicine and Executive VP of Strategic Alliances at EVERSANA. Click here to listen to the podcast.

A Predictive Analytics and Machine Learning Approach to Improving Hub Performance and Patient Outcomes
Today we have access to more data, from more sources than we could ever dream possible. Living in a digital world, we increasingly need the ability to efficiently and effectively process this data for insights and actions in order to be competitive. The life sciences industry can leverage this data using analytic tools and machine learning to rapidly identify patient behaviors and patterns – allowing us to predict “next best actions” in our quest to improve patient outcomes.

Leading and Decision-Making in Uncertain Times
By definition, leaders must have followers, who are defined as people who must be shown the way. While this is within most leaders’ grasps when the road ahead is straight and wide and the destination is visible, more is demanded of them when the road splits, narrows and dusk falls. The current crisis presents special challenges to business leaders who have experienced largely smooth sailing for the longest bull market in history. Used to relying on historical data to project the future and to relatively predictable competitive and regulatory events in the world of operations, pharma’s leaders have focused on addressing more strategic challenges, adjusting corporate capability, capacity and culture […]

5 Trends in Digital Medicine to Watch For
We Are Uniquely Positioned To Deliver EVERSANA is uniquely positioned to follow the trends and introduce new models and strategies to maximize the value of pharmaceutical products through Digital Medicine Solutions and Execution. Digital Solutions can be deployed to impact the entire life cycle of a product in unprecedented ways. Schedule a 30-minute call with our Digital Experts Team to learn more.

Healthcare Pivot: Digital Medicine Delivers During A Global Crisis – Reviewing Commercial Pathways
In February 2020, healthcare as we knew it changed. The industry began waging an aggressive war with an unknown virus beginning to overwhelm hospitals on a global scale. We immediately recognized that we needed to do things differently. Virtual healthcare began to rise, and disruptive advancements in medical technology began breaking down siloed healthcare delivery models and transforming the connectivity between patients and their providers. Digital medicine and telemedicine exploded because the industry pivoted – driven by the increased need for safe and effective healthcare treatments – and embraced digital medicine, an idea that has been quietly growing for more than ten years. Enabling this pivot is a myriad of […]

PharmaVOICE Precision Medicine Showcase
Improving Patient Adherence With Behavioral Health Technology The industry’s shift to outcomes-based, patient-centered care requires new approaches to patient support to achieve adherence and therapeutic success. Today’s patients are also highly motivated information seekers and are taking control of their day-today therapy. And, like all modern consumers, they expect outcomes to be personalized, delivered on their terms, powered by technology, and of high value. In the April PharmaVOICE Precision Medicine Showcase, EVERSANA’s Kathi Henson looks at how patient insights inform a new model of support services for complex therapies, the continuity of rare care, and the economics of patient understanding.

Two Key Questions About Payer Strategies in 2020
In a PM360 Focus on Payer Strategies feature, Brian Davis addresses the question: Besides efforts to lower drug prices and increase transparency, what market access challenges/barriers will cause pharma companies the most stress this year? How can companies overcome this challenge/barrier? In his response, Brian highlights a few of the 2020 challenges to market access, identifies trends, and provides solutions to help pharma companies successfully navigate. Click here to read the article.

PharmaVOICE Showcase on Connected Health and IoT
The Growing Potential of Connected IoT In PharmaVOICE’s Showcase on Connected Health and IoT, Ed Cox offers executive viewpoints on the challenges of integrating the Internet of Medical Things (IoMT) into pharma processes. “Breaking down siloes by supporting technology that provides value across the patient care ecosystem and investing in IoMT that generates data and seamlessly interacts with other systems is game changing for patients and healthcare overall.” He also identifies the benefits of data collected by connected health tools to pharma. Download the PharmaVOICE article.

PharmaVOICE Outsourcing Showcase
The New Roaring 20s: How Outsourced Commercialization is the Revolution We Need. To effectively commercialize a product, traditional service silos stand in the way of true healthcare transformation. Do disparate service providers look at the life cycle in the same way you do? There is a real need for a market access strategy that converts into measurable value, and it’s crucial how everything from pricing and payer outreach informs the 3PL, specialty pharmacy, HUB, and pharmacovigilance services. Most importantly, these services must integrate into a seamless patient experience that’s strong enough to withstand loss of exclusivity or competing products. In PharmaVOICE’s Outsourcing Showcase, Greg Skalicky illuminates how the New Roaring […]

A Patient Hero
Making the Patient an Extension of the Team The concept of patient-centricity is well embedded throughout EVERSANA and is intrinsically tied to helping the agency’s clients achieve their goals. Kristin LaBounty Phillips believes that the entire agency — with a unified commitment to quality in addressing patients’ needs — embodies the concept of “patient hero.” The 2020 PharmaVOICE special Patient VOICE issue featured Patient Heroes – individuals who go the extra mile to improve the patient experience. Kristin discusses her passion for patients, how she is enhancing patient engagement, and embracing patients as partners as part of the process. Read the whole interview.
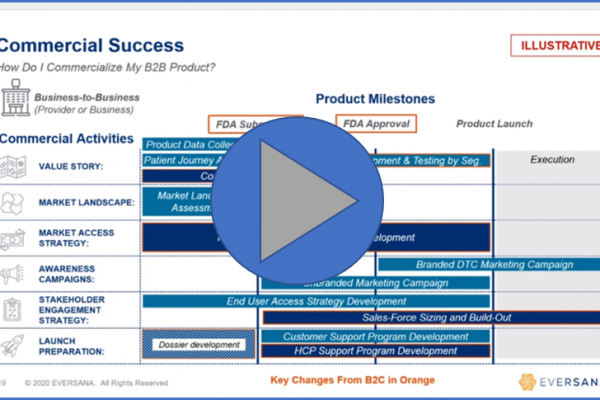
Realizing Value from Digital in the Healthcare Environment [Slides]
[Presentation Slides] The advancements of Digital Therapies are promising but commercial success is not always guaranteed. This presentation explores the pitfalls and challenges of selling products into the uniquely complicated and regulated healthcare market. It will lay the groundwork for digital companies to have successful commercial strategies and be able to: Answer questions like: What is my commercial model? What is my pricing model? How are payers thinking about my disease? Who pays today? Will I get coverage? How does a doctor’s prescription turn into a patient’s download and use? What answers do I need now vs. later? Download the presentation slides below OR watch the 20 minute webinar.

Realizing Value from Digital in the Healthcare Environment [Webinar]
[WEBINAR – 20 MINUTES] The advancements of Digital Therapies are promising but commercial success is not always guaranteed. This presentation explores the pitfalls and challenges of selling products into the uniquely complicated and regulated healthcare market. It will lay the groundwork for digital companies to have successful commercial strategies and be able to: Answer questions like: What is my commercial model? What is my pricing model? How are payers thinking about my disease? Who pays today? Will I get coverage? How does a doctor’s prescription turn into a patient’s download and use? What answers do I need now vs. later? Download the slides OR view the 20 minute webinar.

The New Roaring 20s: How Outsourced Commercialization is the Revolution We Need
The “Great Gatsby Era” was an exciting time of new prosperity, infinite creativity and dramatic social change. Long-standing social norms and traditions gave way to the “mass culture” and consumerism that modernized American society. “What will they think of next?” was a popular expression that defined the era. On the 100-year anniversary of this revolutionary decade, the pharma industry should take note: breaking from tradition is the catalyst for real, long-lasting transformation. Innovative therapies require innovative thinking. In just the past 10 years, we’ve seen both clinical and manufacturing outsourced to single partners who successfully reduced costs, minimized risk, and maintained product integrity. Commercialization, on the other hand, is lagging […]

Trends Disrupting Life Sciences in 2020
From the commercialization of digital medicines to the evolution of value-driven healthcare – our thought leaders deliver insight on the trends impacting the life science industry that will continue to shape our thinking well in 2020 and beyond. Take a look at the trends we’re tracking by clicking on the links below: The Transformative Promise of Digital Health The promise of digital health – from wearables to mobile health apps – could revolutionize healthcare by improving adherence, reducing costs, and making treatments more personalized. ASK THE EXPERT about Digital Medicines A New Partnership in Digital Medicine The Impact of Emerging Technologies Therapies are becoming more targeted to narrower populations. Technology […]

How to Maximize the Value of New Advances in Oncology
Today we have access to more data, from more sources than we could ever dream possible. Living in a digital world, we increasingly need the ability to efficiently and effectively process this data for insights and actions in order to be competitive. The life sciences industry can leverage this data using analytic tools and machine learning to rapidly identify patient behaviors and patterns – allowing us to predict “next best actions” in our quest to improve patient outcomes.

Strategies to Ensure Market Success of a Rare Disease Product Launch
Small patient populations, complex administration, high costs of therapies, and government policy interventions are just a few obstacles in rare disease. EVERSANA’s Managing Director, Bill O’Bryon, and Senior Director, Diann Johnson, outline key insights to consider in your launch strategy: Market dynamics impacting rare disease therapies The entire rare community: patients, caregivers, providers & advocates Payer landscape and engagement Patient experience Stakeholder relationships Watch the Webinar: Strategies to Ensure Market Success of a Rare Disease Product Launch

Ask the Expert – Digital Therapeutics
From reimbursement strategies, distribution, dispensing, patient engagement and adherence programs, EVERSANA offers integrated solutions to alleviate the barriers facing the adoption of digital therapeutics. Mike Ryan, Executive Vice President of Europe and Asia Pacific, discusses how EVERSANA is leading the commercialization of digital therapeutics with an innovative and nimble commercial model valuable to patients, providers and payers at launch.

WHAT IF… We Broke the Silos Throughout Pre- to Post-Commercialization?
In their special November/December issue, PharmaVOICE asked industry leaders to think beyond the status quo and provide insight into what needs to happen to achieve aspirational goals and evolve the healthcare industry in 2020. In the feature article titled, What if…” PharmaVOICE turns to their community of thought leaders to weigh in on their own questions. Topics ranged from funding for innovation to unlocking the value of healthcare data and achieving data interoperability, of HEOR informing all healthcare decisions and patients being the ultimate decision makers in the drug development process, to living in a world without disease. EVERSANA’s Greg Skalicky, Chief Revenue Officer, shares his insight on the impact […]

After FDA Approval, Can Gene Therapies Achieve Marketing Success?
Without a doubt, gene therapy is transforming healthcare by revolutionizing patient care from conventional treatment models to curative therapy models. There are more than 7,000 distinct types of rare and genetic diseases and 400+ million individuals suffering from a rare disease. With a market that is fragmented, there is a need for an innovative, end-to-end commercial solution to support these new therapies and lead the pharmaceutical product lifecycle from clinical trial recruitment though commercialization. In this point of view published in the special November/December 2020 Preview issue of PharmaVOICE, Greg Skalicky, Chief Revenue Officer, discusses what is needed for gene therapies to succeed after FDA approval. To read the article […]

Innovative Therapies Call for an Integrated Drug Safety and Compliance Model
In this article, Herbert Lee, PharmD, Senior Vice President, Medical Communications and Pharmacovigilance, makes the case for an integrated approach to ensuring the safe and effective use of medications by patients. From emerging therapies to innovative technologies, the healthcare industry is changing – demanding that we do business differently. Working in one of the most regulated industries, we need to understand the complexities of developing products/services that meet patient need and help improve the health and safety of the public. Safety and compliance are important areas of focus in every industry I can think of and especially in life sciences. As the global environment evolves, so must our models for […]

Building a Service Offering from the Research Lab to the Patient’s Home
A Conversation with Pharmaceutical Commerce & Jim Lang, Chief Executive Officer, EVERSANA Not quite a year ago, an assemblage of pharma service providers came into being as EVERSANA. Funded by two private equity firms with a background in healthcare—Water Street Healthcare Partners and JLL Partners, there were six acquisitions at the time: Dohmen Life Science Services (itself a prior rollup of contract logistics, rare disease patient support and specialty pharmacy), The Access Group, Alliance Life Sciences, Health Strategies Group, Triplefin, and Patient Experience Project. Shortly after, it added Seeker Health and field sales units acquired from Mission Pharmacal. Together as EVERSANA, the organization delivers a fully-integrated service ranging from clinical […]

Are We Transforming In The Right Way?
Why Product Launches Can’t Be Distracted By Empty Promises. We can all agree that innovations in therapeutic development have advanced beyond traditional product launch strategies and service models. In every step of the product lifecycle, we see pockets of transformation. The problem is exactly that – “pockets” of transformation. Your product launch strategy has to achieve the following: Value the voice of the patient Protect your revenue Reach all stakeholders now Prepare for optimal distribution Achieve broad and quality access by reaching all stakeholders In this point of view published in the September issue of Pharmaceutical Executive, Greg Skalicky, Chief Revenue Officer, argues that traditional service silos stand in the way […]

Four Key Questions About Patient Experience in 2019
In this September feature, PM360 asked experts for insight into the changes that need to happen to create better experiences for patients and ultimately serve them better. Kathi Henson, Chief Patient Service Officer, shares insight into how the increased access to real-time patient data helps life sciences companies better understand patients and improve their experiences. From better key opinion leader engagement through HEOR and RWE research to communicating value to payers to ensure market access, Kathi highlights key data points that drive successful patient support programs. To read Kathi’s response, click here.

EVERSANA, Patient Experience Project featured in MM&M July Issue
Each year, Medical Marketing & Media compiles a list of the top 100 medical marketing agencies in North America. As MM&M Editor Stephen Madden shares at the opening of the July issue, “A typical issue of MM&M has 56 pages; with 244, this beast has almost five times as many.” From agencies A to Z, the issue is complete with profiles on the top 100 agencies. It also includes a key article, “New Breed Networks are Reimagining the Agency Offering,” featuring commentary from EVERSANA’s CEO, Jim Lang. You can find the article on page 10 of the issue. Page 140 features the profile on the Patient Experience Project, an EVERSANA […]

Gene Therapy for Rare Disorders
With only three approved products in the marketplace, how do we build a regenerative medicine ecosystem that delivers more value to patient’s faster?

Setting a Patient Engagement Framework and Proving Value
Is your culture patient-centric? It needs to be because today’s patients are motivated information seekers – empowered decision-makers with respect to their treatment. Their voice is key to your success.

Insights from AMCP 2019
EVERSANA attended (and sponsored) The Academy of Managed Care Pharmacy (AMCP) Managed Care & Specialty Pharmacy Annual Meeting. If you missed the conference, here are a few key takeaways from our colleagues who attended: 1) Federal and State Legislature Update Increases in federal and state policies are putting more pressure on drug prices The possible removal of some Safe Harbor laws and Anti-Kickback statutes “Big Data” is increasing access to patient data; data ownership is still an issue (Key question: Who owns the data?) There was a big emphasis around future trends and the role big data will play. And, Artificial Intelligence (AI) and virtual medicine were recurring themes throughout the meeting […]

Providing a Patient-Driven Experience
As the “patient-as-consumer” culture enables patients to consider all their varied care options, they increasingly want to be involved not only by participating in clinical trials, but also by accelerating product development and the availability of patient support programs after a product reaches the market for a rare and complex disease. And, like all modern consumers, they expect outcomes to be personalized, delivered on their terms, and of high value. And in our roles as marketers, we are also now responsible for delivering value across the entire product life cycle. This requires a strategic and coordinated approach that includes patient-driven data analysis and innovative research techniques to generate real insights […]

Sell’N Gene Therapies: A Landscape Assessment Of Cell And Gene Therapy Reimbursement
Cell and gene therapies hold great life-saving potential, with the ability to treat disease states with significant morbidity, mortality, or treatment-related complications. Kymriah became the first CAR-T cell therapy to bring transformative efficacy to B-cell acute lymphoblastic leukemia in children and young adults in August 2017. Luxturna—the first-ever treatment for a rare form of hereditary blindness—also became the first in vivo gene therapy approved in the US in December 2017. By some estimates, as many as 40 or more cell and gene therapies will launch in the next five to seven years. In addition to bringing substantial benefits to patients, many of these new therapies will bring steep price tags. While […]

Put Your Patient Services Program to the Test
How well do you really know your patients? Global Genes reports that therapy adherence in the rare disease space can vary from 58 to 65 percent, a troubling statistic for our industry, and most importantly, the patients we serve. Manufacturers must be wondering, how well do we really know our patients and what they need? Usha Roy invites you to take this brief quiz to see if your program answers important patient-centric questions. Usha leads patient support teams focused on implementing and improving patient access and adherence programs to ensure patients overcome barriers to therapy, achieve higher adherence, and receive the best possible care. Download the paper to answer the […]

Will the U.S. adopt global reference pricing?
This article was written for Med Ad News Magazine There are many things that Americans get from outside our U.S. borders. Our top imports include oil, machines, and cars; our iTunes and Spotify accounts are stocked with The Beatles, U2, and Ed Sheeran; and you practically can’t go to any city in America without being able to order a pizza, tacos, or sushi. Although it is true that our country has adopted many things that have improved our lives from outside the U.S., one thing we have not made efforts to adopt is how pharmaceutical drugs are priced outside of the U.S. – that is, until now. Read the […]

Product Master Data Management
This white paper shares considerations, common pitfalls and key takeaways for manufacturers participating in Medicaid. Recent market and enforcement trends in healthcare further exemplify the transition from a volume to value-based marketplace, as well as the complexities to contain the increasing costs of drugs in the new paradigm. The costs of branded prescription drugs are rising at an alarming rate that is unsustainable. Agency enforcement efforts highlight that more authority is needed for imposition of penalties and corrective actions to recover over payments under Medicaid. Concurrently, legislation enacted, and regulatory changes continue to target cost containment of prescription drugs subsidized by Medicaid and Medicare. Under the Medicaid Program, these changes have […]

The Radical Implications Of Indication-Specific Pricing
This paper discusses one of the potentially more impactful new dynamics in the pricing of pharmaceuticals—the advent of indication-specific pricing. Our goal is to provide context to this important change, highlight some of the implications about which biopharma companies should be aware, and leave you with a framework for raising and addressing pertinent questions across the biopharma value chain. A major change is under way that has the potential to impact the entire process of developing, pricing, and selling pharmaceuticals. Express Scripts, a leading pharmacy benefit manager (PBM) and pioneer in drug cost management, has introduced an indication-specific pricing model, whereby drugs are reimbursed differently based on their effectiveness in […]

Don’t Poke The Bear: The Effect Of Pharmaceutical Pricing On Perception And Future Innovation
Companies like Turing Pharmaceuticals and KV Pharmaceuticals have grabbed headlines recently for their aggressive pricing strategies. Whether these strategies are justified or even successful is not the point. Rather, what is important is the negative public perception that significant price hikes create for the industry as a whole. In this climate, the industry needs to do a better job of talking about how prices are set and how that money is invested—otherwise we risk losing control of how we price our products and operate our businesses. Download the full White Paper.

