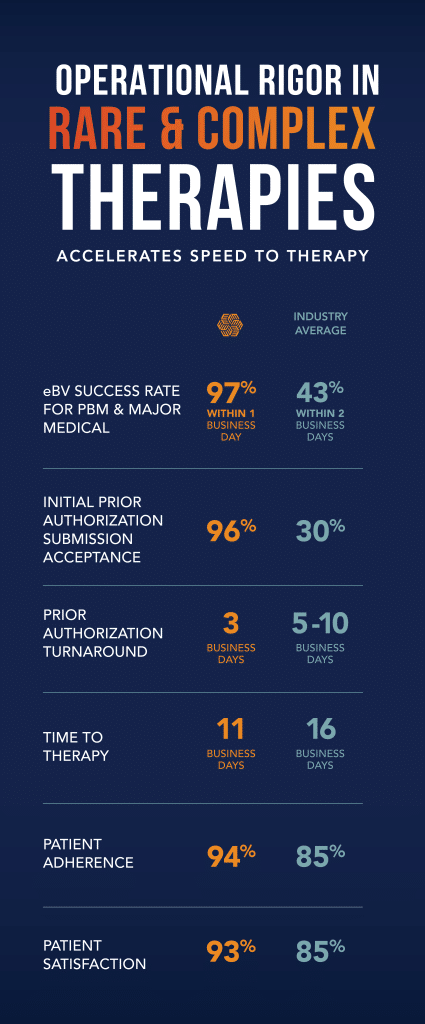
Critical Success Factors for Launching Products with Orphan Drug Designation

The Orphan Drug Act (ODA) was passed in 1983 to financially incentivize pharmaceutical companies to develop drugs for rare diseases or conditions, defined as a disease or condition that affects less than 200,000 people in the US.1 The financial incentives offered by the ODA are substantial and include tax credits up to 50% of the qualified clinical testing expenses for the taxable year, waived Prescription Drug User Fee Act (PDUFA) user fees that may be upwards of $3 million in 2022, and an extended market exclusivity period of seven years after approval.2 A recent analysis found that receiving an orphan drug designation does, on average, positively impact companies’ value as […]