Navigating Routes to Commercial Excellence in Cell and Gene Therapy

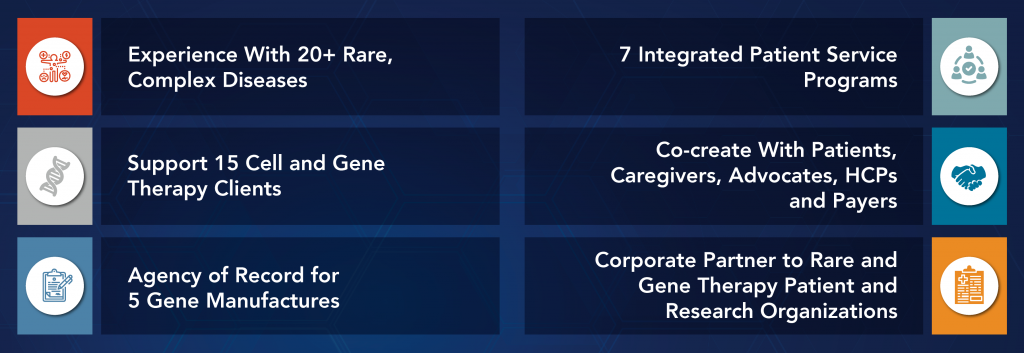
“Navigating Routes to Commercial Success in Cell and Gene Therapy” discusses the unique challenges faced by companies developing and commercializing cell and gene therapies (CGTs). The paper highlights the significant potential of CGTs in treating previously incurable diseases and the need for a tailored approach to commercialization given the complexity of these therapies, high development costs, and regulatory hurdles. It provides an overview of the current market landscape and emerging trends in CGT development and commercialization, including the importance of real-world evidence, patient engagement, and pricing and reimbursement strategies. The paper also outlines best practices for successfully navigating the commercialization journey, including the importance of a cross-functional approach, strong data […]