Insights
Insights From Our Experts
Articles

Develop Patient Support Programs Focused on Patients
We have to get intimate with patient populations and advocacy groups to build best-in-class patient support programs from the start.

Providing a Patient-Driven Experience
As the “patient-as-consumer” culture enables patients to consider all their varied care options, they increasingly want to be involved not only by participating in clinical trials, but also by accelerating product development and the availability of…

Sell’N Gene Therapies: A Landscape Assessment Of Cell And Gene Therapy Reimbursement
Cell and gene therapies hold great life-saving potential, with the ability to treat disease states with significant morbidity, mortality, or treatment-related complications. Kymriah became the first CAR-T cell therapy to bring transformative efficacy to B-cell acute…

Put Your Patient Services Program to the Test
How well do you really know your patients? Global Genes reports that therapy adherence in the rare disease space can vary from 58 to 65 percent, a troubling statistic for our industry, and most importantly, the…
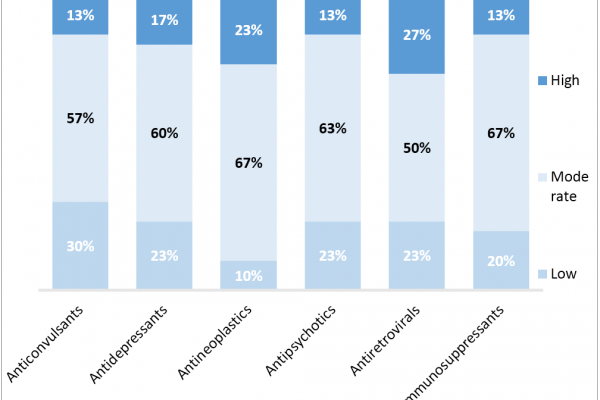
How Could Policy Changes to Protected Classes Impact Part D Access and Contracting?
(Content Updated 5/17) CMS Protected Class Rule Finalized, Slightly Loosened for Biopharma Following CMS’ 2018 proposed rule allowing for new protected class exceptions and a comment period for stakeholders through the beginning of this year, CMS…

Will the U.S. adopt global reference pricing?
This article was written for Med Ad News Magazine There are many things that Americans get from outside our U.S. borders. Our top imports include oil, machines, and cars; our iTunes and Spotify accounts are stocked…

Pathways for Paying for Rare Disease Treatments
This article was written for the Journal of Clinical Pathways Determining how to pay for the treatment of uncommon yet serious diseases is an important consideration in terms of sustainability and patient access. Novel and expensive…
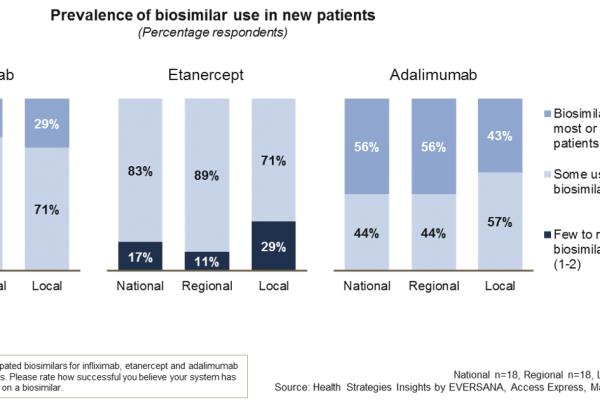
New start versus switch patients for biosimilars?
Over the last several years, European market demand for biosimilars has accelerated with the entry of biosimilars for many reference biologics, including high-value molecules such as adalimumab, infliximab and etanercept. However, there have been reported variations…
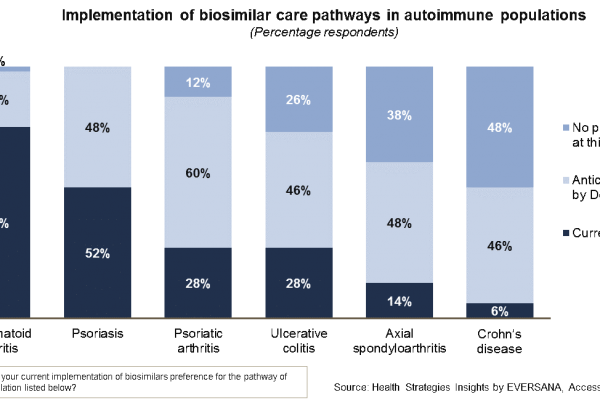
Care pathways—do you know where your biosimilars are used?
Biological therapies are often very expensive, putting pressure on healthcare budgets that are already restricted and potentially resulting in a decrease in patient access to treatment, in Europe biosimilar versions have been eagerly awaited in many…
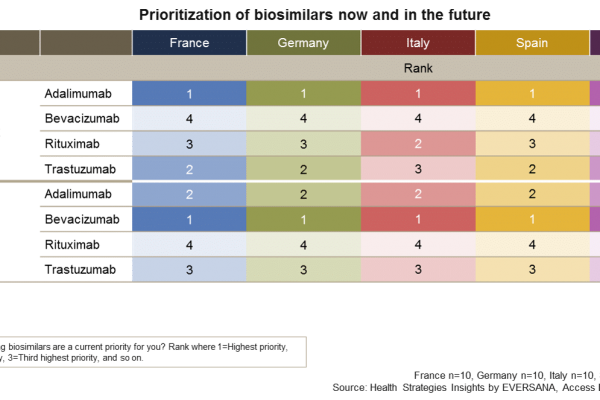
Is your biosimilar product on the payer radar?
Biosimilars have been eagerly awaited in many European countries to realize cost savings from the biologics budget. Gaining insight on how European decision makers are currently prioritizing these biosimilars and how they expect this to shift…

