Insights
Insights From Our Experts
Articles

340B Drug Pricing: Challenges and Best Practices
Enacted in 1992, the 340B Drug Pricing Program was intended to stretch scarce federal resources and services to more eligible patients. Despite achieving this goal in many respects, it has also become notoriously difficult to…

Pharmaceutical Contracting from Volume to Value
Driven by skyrocketing costs, market forces are hastening the shift in the healthcare industry from volume based care (fee for service) to a value based reimbursement structure (fee for value). While the inertia of entrenched policies,…

Gene Therapy for Rare Disorders
With only three approved products in the marketplace, how do we build a regenerative medicine ecosystem that delivers more value to patient’s faster?

Insights from AMCP 2019
EVERSANA attended (and sponsored) The Academy of Managed Care Pharmacy (AMCP) Managed Care & Specialty Pharmacy Annual Meeting. If you missed the conference, here are a few key takeaways from our colleagues who attended: 1) Federal and…

Sell’N Gene Therapies: A Landscape Assessment Of Cell And Gene Therapy Reimbursement
Cell and gene therapies hold great life-saving potential, with the ability to treat disease states with significant morbidity, mortality, or treatment-related complications. Kymriah became the first CAR-T cell therapy to bring transformative efficacy to B-cell acute…

Pathways for Paying for Rare Disease Treatments
This article was written for the Journal of Clinical Pathways Determining how to pay for the treatment of uncommon yet serious diseases is an important consideration in terms of sustainability and patient access. Novel and expensive…
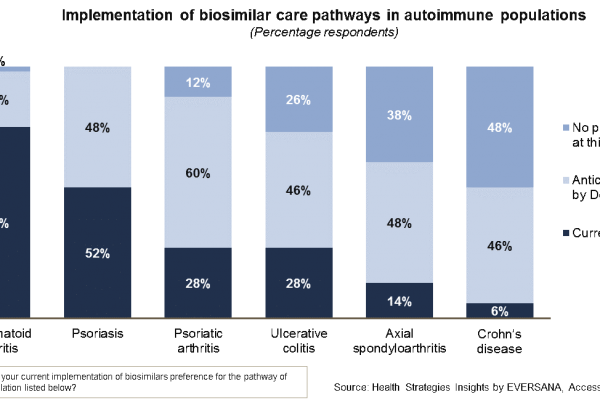
Care pathways—do you know where your biosimilars are used?
Biological therapies are often very expensive, putting pressure on healthcare budgets that are already restricted and potentially resulting in a decrease in patient access to treatment, in Europe biosimilar versions have been eagerly awaited in many…
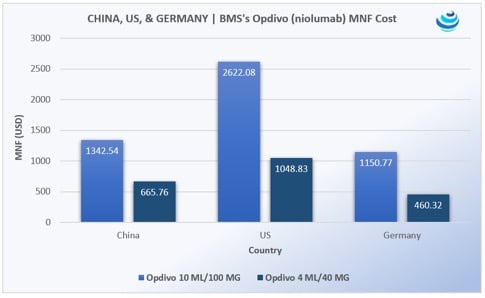
‘Tis the season for pharma in China, as the government expedites uptake of new urgently needed drugs
PRICENTRIC BRIEF: Universal health insurance coverage for 1.3 billion people means China must increase uptake of “clinically urgently needed new drugs” already approved in the US, EU, and Japan Mostly oncology products are being imported, along…
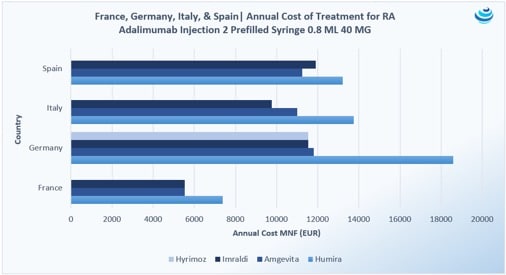
“Who wants the biggest slice of the biosimilar pie?”: The Humira biosimilar wave in Europe
PRICENTRIC BRIEF: Biosimilar competition in Europe has brought about discounts to AbbVie’s blockbuster immunosuppressant drug Humira upwards of 80% during tendering Overall, biosimilar uptake has increased in Europe because biologic “copycats” are cheaper, but full faith…

Medicare & Medicaid: A Rollback on Rebates
In January 2019, the Department of Health and Human Services (HHS) put forth a proposed rule to repeal the safe harbor status for Medicare and Medicaid drug rebates under the Anti-Kickback statute. This idea was first…

