The FDA’s draft guidance on Prescription Drug Use-Related Software (PDURS) offers pharmaceutical manufacturers a novel strategy to enhance a drug’s label by enabling an HCP to prescribe software alongside the drug at the HCP’s discretion. The new framework makes it easier than before to pair software with a medication to help patients manage complex dosing, monitor side effects, and access behavioral change support.
For pharmaceutical companies, the benefits of developing a PDURS product include the potential for brand differentiation, improved patient adherence, and demand retention post loss of exclusivity (LOE). PDURS also unlocks a new go-to-market option for digital health software.
Released in September 2023, the PDURS draft guidance details regulatory considerations for adding software outputs to a drug’s label. Software outputs that have demonstrated meaningful clinical benefit would be added to Section 14 “Clinical Studies” of the label’s Prescribing Information.
By making it easier to combine software with a pharmaceutical product, PDURS opens the door to more of such pairings. In this article, we will explore the regulatory and clinical requirements of PDURS and the possible commercial impact manufacturers might expect from taking this exciting new approach.
Regulatory and Clinical Requirements
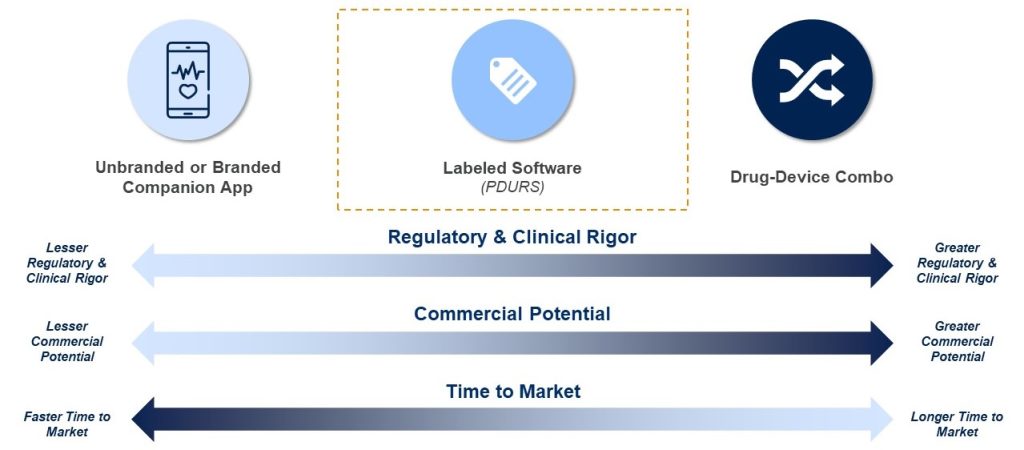
In the absence of the new PDURS framework, digital devices can be paired with drugs via two pathways: companion software and drug-led combination products. Each of these approaches provide their own advantages and challenges.
Companion software, either branded or unbranded, has limited regulatory rigor. These solutions generally are not subject to regulatory oversight by FDA’s Center for Devices and Radiological Health (CDRH) and can be developed in relatively short timeframes and deployed alongside a drug. Branded companion software has an additional regulatory requirement that it must be submitted as promotional labeling to FDA’s Office of Prescription Drug Promotion (OPDP), but this process is relatively straightforward and there is no approval process. The companion software pathway has the advantage of speed to market and does not require clinical validation nor burdensome medical device quality system requirements. However, manufacturers are only able to make modest marketing claims that do not claim a medical purpose. Anti-kickback laws also prevent HCPs from providing anything of value to patients freely, limiting usage of digital companions in practice. In many cases, these software solutions are focused on patient support or are promotional in nature and have limited adoption and commercial impact.
In contrast, in a drug-led combination product, the device is, by definition, essential to the intended use of the drug. These solutions must be rigorously tested, clinically validated, typically with a randomized controlled trial, and submitted to the Center for Drug Evaluation and Research (CDER) as a New Drug Application. CDRH is consulted for the review of the device portion of the combination product. As a result of the clinical and regulatory rigor, combination products require a significant investment of time and resources to bring to market, and also can present significant risk to the drug if it is already on market since it must be tested in a new Phase 3 study. For these reasons, there is not yet a single example of an FDA-approved drug-led combination product where the device constituent is standalone software (i.e., there is no hardware). However, a combination product would have more commercial value because the FDA acknowledges the value of the software in the context of the drug, and the manufacturer is able to make marketing claims that promote the clinical benefit of the software to the patient population.
The PDURS framework for FDA-required labeling is an alternate approach that FDA introduced using existing regulations and policies. As we outlined in an earlier assessment prior FDA’s introduction of the guidance, this approach is explicitly intended to foster innovation and provide manufacturers with added flexibility to develop and disseminate new software. The framework offers a middle ground between the two existing pathways described above, providing greater commercial benefit than a companion product, but with fewer regulatory hurdles than a combination product and less risk to the drug.
According to the new draft guidance, software that has demonstrated meaningful clinical benefit to a drug, whether through improved safety or efficacy, is regulated by CDER as FDA-required labeling and can be added to the drug label through an NDA supplement rather than a new NDA. Well-controlled studies are required to demonstrate a clinically meaningful benefit, per the draft guidance. Our interactions with the Agency have clarified that a risk-based approach will be taken in considering the type of evidence and control condition. Most companion apps have low to minimal risk, and we anticipate that well-designed real-world Phase 4 studies could suffice for certain intended uses.
Note that the CDRH regulatory framework remains in place, so the manufacturer must consider both the PDURS framework and CDRH’s oversight of software as a medical device (SaMD). In all cases, manufacturers should engage with the Agency early in the process to ensure that the proposed labeling and evidence generation plan are aligned to CDER’s expectations.
Commercial Impact of PDURS
The commercial impact of PDURS is secondary to the clinical impact. Above all, a PDURS offering must demonstrate meaningful clinical benefit to be included on the drug label. Once that is demonstrated, the commercial potential for PDURS can be unlocked. Some clinical use cases for PDURS include, but are not limited to:
- Dose optimization,
- Side effect monitoring and management,
- Behavioral health support and
- Prognostics for disease severity and flares.
Digital solutions have been useful in predicting likelihood of side-effects. For drugs with serious side effect profiles, a PDURS solution can provide utility in mitigating some of these adverse events by capturing or predicting an adverse event and recommending an intervention or escalation. Additionally, for drugs that require a period of time before its intended effect, PDURS products can help patients remain on drug until outcomes are seen.
Ultimately, PDURS can be influential in supporting patient retention, which in turn can improve patient outcomes and brand performance.
But when should PDURS be considered? There are three key factors that should influence PDURS consideration:
- The drug’s clinical profile,
- The level of market competition and
- When the drug is expected to lose exclusivity.
A PDURS product can also add another level of differentiation against competition. For consumer-focused therapeutic areas, it can be an attractive offering alongside a drug to encourage patients to ask for the brand by name. While adding PDURS does not affect the molecular entity and will not extend patent life of the drug, developing a novel PDURS product can create another layer of differentiation that generic manufacturers will be unable to reproduce.
Developing a PDURS product in these scenarios yields commercial benefits that include, but are not limited to, brand differentiation, patient adherence and demand retention.
Brand Differentiation: For products in highly competitive markets, PDURS can be a strategy for drugs to overcome clinical shortcomings and bolster their efficacy, safety, and overall value proposition.
Patient Adherence: Adherence has been a challenge for pharma, and with patient outcomes so closely tied to adherence to treatment, various strategies have been pursued to ensure patients stay on medications for optimal outcomes. Leveraging the PDURS framework, manufacturers can employ a variety of strategies to increase retention.
Demand Retention: Maintaining market share post LOE is always a challenge. PDURS offers a value-added, product differentiation strategy to help dampen the effects of loss of exclusivity.
In short, this framework creates options for a prescriber, allowing prescriptions of PDURS to be written as the HCP sees fit.
Conclusion
The PDURS framework offers an exciting new way to leverage digital assets alongside existing treatment options and has the potential to positively impact patients’ lives in various ways. Manufacturers looking for ways to integrate digital into their offerings or facing any of the scenarios outlined above should consider several important questions:
- Which portfolio products are candidates for PDURS?
- How can PDURS solutions be designed to augment a brand’s performance?
- What level of evidence is required for a PDURS solution?
- What is the best way to engage with the FDA to advance a PDURS solution?
To assist in answering these questions, EVERSANA has a deep network of industry professionals with regulatory, commercial, and digital solutions expertise. We’re ready to help any organization to harness the true power of PDURS.
Author

John Kutz, Senior Partner at EVERSANA MANAGEMENT CONSULTING, is a life sciences professional who has spent the last 20 years of his career helping clients launch new products, develop leading-edge marketing capabilities, and develop…

Marty Culjat, PhD is the SVP, Global Head of Digital Medicine & Regulatory Innovation at EVERSANA. In this role, he leads a cross-functional team supporting the commercialization of digital medicine products within companies ranging…

Justin Averback is an Associate Partner at EVERSANA MANAGEMENT CONSULTING, with experience across the development lifecycle, including product commercialization, market research, project management, and brand strategy. With EVERSANA MANAGEMENT CONSULTING, Justin has collaborated with…

Calvin is a Consultant at EVERSANA MANAGEMENT CONSULTING, possessing industry experience across several therapeutic areas, with an emphasis in forecasting, data analytics and digital therapeutics. Prior to joining EVERSANA, Calvin was a Consultant with…

Joey is a Consultant at EVERSANA MANAGEMENT CONSULTING with applied life sciences industry experience and scientific research experience. While at EVERSANA, Joey has worked on a number of forward-looking commercial and innovation strategy projects…

