
NAVLIN Insights Team
NAVLIN Insights gives you an advanced set of capabilities, frameworks, and methods. These are all derived from a robust stakeholder panel, providing critical answers to your specific payer marketing and market access questions.
NAVLIN Insights gives you an advanced set of capabilities, frameworks, and methods. These are all derived from a robust stakeholder panel, providing critical answers to your specific payer marketing and market access questions.
Articles by NAVLIN Insights Team

Delivering Value to Leading Payers
Assessment of Strategic Priorities, Competitive Dynamics, and Innovations Impacting Biopharma Access Seven leading payers represent nearly half of all US medical lives and 54% of pharmacy lives: UnitedHealth Group CVS Health, Cigna Elevance Health Kaiser Permanente Humana Centene As these organizations evolve their business models and ultimately their approaches to care and drug management, biopharma […]
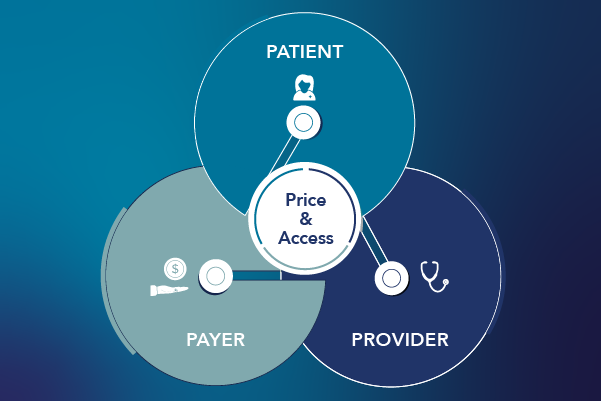
Informing Optimal Pricing Strategies with a Comprehensive Modeling Approach
Strategically pricing new therapies has always been vitally important for pharmaceutical manufacturers. After the 2022 passing of the historic legislative package, known as the Inflation Reduction Act (IRA), strategic pricing strategies have become especially pivotal. The IRA gives manufacturers less flexibility to adjust their pricing strategies due to penalties that are now applied for price […]

The Influx of Generic Launches Leaves Narrow Opportunities for Multiple Sclerosis Access
Since the launch of Sandoz’s Glatopa in June 2015, generic options have slowly entered the multiple sclerosis market, providing some cost relief to payers in this high-cost market. Generic entrants, along with rising costs and a perceived lack of differentiation among brands on key clinical outcomes, solidify multiple sclerosis as one of the most extensively […]

Case Study: Primary Research Meets Data-Driven Sources and Technology to Form Powerful Market Access Solutions
The case for primary market research, Real-World Data (RWD), and deep healthcare experience combined to deliver targeted market access strategies. As diseases become more complex and market access becomes more restrictive, with multiple sources of market research and data analytic solutions available, pharmaceutical executives are often overwhelmed with various sources of information. Leaders must learn […]

European Commission’s Long-awaited Pharma Legislation Review Gets Mixed Reception
NAVLIN Brief The European Commission has finally released its proposal to revise the European Union’s pharmaceutical legislation. The document includes proposals for a new Directive and a new Regulation, which revise and replace the existing pharmaceutical legislation via the largest reform in over 20 years The Commission proposes new incentives for AMR-related products, greater transparency […]

Left-Right-Left: The Latest in Pharma-Payer Ping Pong
Biopharmaceutical companies have offered financial assistance for commercially covered patients’ out-of-pocket (OOP) cost sharing for 20+ years. However, in the ongoing evolution of controlling versus encouraging patient access to specific drugs, payers and biopharma also have a long history of one-upmanship. Are pharma motivations for offering copay assistance centered on the patient, their own bottom […]

Your Guide to Developing a Best-in-Class Specialty Pharmacy Network Strategy
Why Develop a Specialty Pharmacy Network Strategy? Specialty pharmaceuticals often require high-touch, labor-intensive services to expedite patient access and improve therapy adherence. Additionally, patients requiring specialty therapies are often medically complex and challenging to manage. These factors highlight the need to identify SP network partners with the proper expertise to achieve optimal product distribution and […]
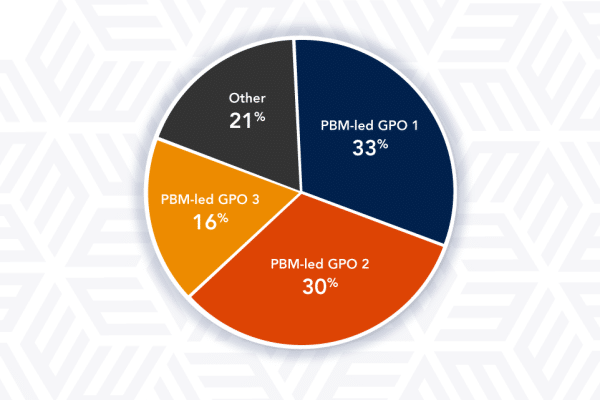
Peeking Behind the PBM-lead GPO Curtain
‘PBM-lead GPO,’ a Newly Created Term to Describe Large Scale PBM-Owned Rebate Aggregators PBM-led group purchasing organizations (GPOs) are not true group purchasing organizations in scope, nor are they pure rebate aggregators. Rather, PBM-lead GPOs are organizations formed by PBMs with one or more members, whose primary purpose is to aggregate purchasing volume to negotiate […]

Ready or Not, Biosimilar Adalimumab Brings the Heat in 2023
Anticipated Growth in U.S. Biosimilars Market U.S. payers and other healthcare stakeholders have eagerly awaited the launch of biosimilar alternatives to high-cost originator products as a new cost-control lever. However, much of the U.S. healthcare market has been frustrated by the sluggish incorporation of biosimilars into the marketplace, as compared to the notably smoother process […]

Case Study: Benchmarking Pathway-Specific Engagement with Health Plans & Organized Providers to Identify Best Practices
Tapping into primary market research and subject matter expertise can help uncover opportunities to more effectively meet their needs and position your brand for inclusion within their pathways. As stakeholders seek to leverage oncology clinical pathways as a way to maximize patient outcomes and decrease costs, biopharmaceutical companies with pipeline and branded oncology drugs must […]

Payer Oncology Access Management in 2022
Payers balance the severity of disease with the need to control high-dollar utilization. As pharmacy spend continues to rise, payers are seeking ways to manage costs in the highest cost therapeutic areas, including oncology. While there is appetite to manage access for both oral and office-administered oncology agents, payers struggle with which tactics to implement without sacrificing patient […]

Developing Best-in-class Copay Programs for Gross-to-Net
Importance of Copay Assistance Programs A copay assistance program is an invaluable strategic cost-saving tool that biopharmaceutical companies integrate into their patient services offering. These programs are used to support a brand’s value proposition, while offsetting high-priced branded drugs, all of which can be the ultimate differentiator for a biopharmaceutical company. Copay programs are designed […]

Five Trends That Will Shape the Current and Future Payer Market Landscape
As leading payers build new business models to ultimately advance their approaches to care and drug management, biopharmaceutical companies need to adjust their account engagement and drug access strategies accordingly. Here are a the top five healthcare trends and payer tactics that our Health Strategies Insights team is watching. 1. Horizontal membership growth for business […]

COVID-19’s Impact on Brand Access
The current public health pandemic is impacting healthcare stakeholders from doctors and nurses in the frontlines to payers and PBMs. EVERSANA™ is monitoring the constant changes to access and benefit consideration, tracking payer responses, and key trends that may impact your brand access strategy. According to recent research released by Health Strategies Insights by EVERSANA™, […]

Brand Access Marketplace Dynamics: Respiratory
This summary provides highlights from robust longitudinal insights reports released throughout the year and available at our INTELLICENTER® portal. The Brand Access Marketplace Dynamics reports identify current and future access landscapes providing insights to support effective identification of opportunities and risks for Respiratory brands. Get answers to key questions such as: What level of influence […]

Brand Access Marketplace Dynamics: Multiple Sclerosis
This summary provides highlights from robust longitudinal insights reports released throughout the year and available at our INTELLICENTER® portal. The Brand Access Marketplace Dynamics reports identify current and future access landscapes providing insights to support effective identification of opportunities and risks for Multiple Sclerosis brands. Get answers to key questions such as: What level of […]

Brand Access Marketplace Dynamics: HIV
This summary provides highlights from robust longitudinal insights reports released throughout the year and available at our INTELLICENTER® portal. The Brand Access Marketplace Dynamics reports identify current and future access landscapes providing insights to support effective identification of opportunities and risks for HIV brands. Get answers to key questions such as: What level of influence […]

Brand Access Marketplace Dynamics: Autoimmune
This summary provides highlights from robust longitudinal insights reports released throughout the year and available at our INTELLICENTER® portal. The Brand Access Marketplace Dynamics reports identify current and future access landscapes providing insights to support effective identification of opportunities and risks for Autoimmune brands. Get answers to key questions such as: What level of influence […]
ACCESSEXPRESS® by EVERSANA Payer Coverage and Reimbursement Case Study
ACCESSEXPRESS allows you to have full control of the survey process getting answers to your most pressing business questions within hours. See how our client used our research platform to assess payer coverage and reimbursement for an upcoming indication expansion. Learn more about ACCESSEXPRESS® by EVERSANA! Fill out the form below to download the Case […]

Oncology Market: Outlook of Brand Access and Insights
The oncology market is constantly changing and EVERSANA continues to assess current and future market trends, payer willingness to pay and the optimal patient support services to ensure you have all the insights you need to answer your most critical business questions. In this summary, our Health Strategies Insights team provides highlights from multiple reports […]

Validate and Inform your Account Access Strategies and Tactics
With $250 million in product revenue, our client is the U.S. subsidiary of a European niche biopharmaceutical company looking to build its presence in the U.S. marketplace in anticipation of future product launches. See how the Health Strategies Insights by EVERSANA Team helped the client put an account strategy in place for each key payer […]

Autoimmune Patient Services Best Practices
Autoimmune Patient Services Best Practices provides highlights from an annual report, available in conjunction with a live, interactive database, updated quarterly which helps to identify patient services best practices by users (e.g., prescribers, office staff and patients) which gives insights into the most valued and highest rated patient services for brands across the autoimmune market. […]

Improving the Impact of an Oncology Patient Access Program
Our client, a specialty pharmaceutical company focused on the research and development of new therapies in two high-cost complex drug categories, sought to benchmark its patient assistance program against those of its competitors in order to improve relationships within target oncologists’ offices. The Challenge The client needed to assess the image of its patient assistance […]

U.S. physicians disagree with Germany’s determinations of the value of diabetes medicines
According to a recent study from Health Strategies Insights by EVERSANA, 89% of U.S. physicians disagree with Germany’s determinations of the clinical benefit of innovative diabetes medicines. Learn more about our Market Access products & capabilities! Contact us with your questions and data needs, and an expert will follow up shortly.

What’s next for ICER? Pharmaceutical pricing watchdog to venture beyond the pill.
Earlier in the month, the Institute for Clinical and Economic Review (ICER) issued a call for public input to help identify and prioritize non-drug topics for ICER assessments in 2020. It may seem like the ICER has always been focused on driving down the price of drugs, conducing health technology assessments to properly calculate the […]

Developing an Optimal Hub to Ensure Product Access
Prior to launching a new Hub, our client, a global biopharmaceutical company, needed to ensure that the services offered would be superior to those of other companies and ensure their product’s access in an increasingly competitive category. The Challenge Our client’s product was one of only a handful of FDA-approved products in a specific category. […]

Decoding ICER: Successful Pharma Engagement with ICER for U.S. HTA Review
How pharmaceutical and Biotech companies engaged ICER drives the outcome. Reactive responses to ICER review have led to poor outcomes and numerous downstream impacts to payer access and pricing. Cohesive enterprise engagement has demonstrated significantly better recommendations and less pressure for the US payer community. Unfortunately, ‘reactive-response’ is more common than ‘enterprise engagement.’ KEY SUCCESS […]

Integrated Delivery Networks: Learning to Engage With a New Customer With Very Different Needs
As the healthcare provider landscape rapidly evolves to deliver on the promises of value-based care and efficiencies of scale, integrated delivery networks (IDNs) have emerged as a dominant force in the US market. Meanwhile, biopharmaceutical manufacturers have struggled to understand the needs of IDNs and how to engage with them in a meaningful way. Leveraging […]

Global Biosimilar Competitor Assessment – Who Wins in Biosimilar Access and Why?
The introduction of biosimilar products has created interesting changes in payer behavior across the global markets. For both innovator and biosimilar companies, it has been an effort to understand the demands of payers and succeed in what looked like a ‘winner-takes-all’ opportunity. As the first round of biosimilar introductions has passed, there are some interesting […]

German and United Kingdom Physicians Express Frustration with Health System Challenges
This brief by Health Strategies Insights summarizes preliminary results compiled from twelve 30-minute, one-on-one, in-depth, telephone interviews with a mix of physician specialists from the United Kingdom (U.K.) and Germany. While the physicians discussed benefits of their respective health care systems, many raised significant challenges and limitations in providing the care they believed would be […]
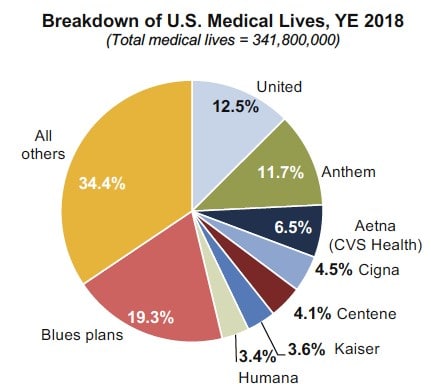
Power in numbers: Seven national plans manage almost half of the medical lives in the U.S.
Market Access Macrotrends provides insight for you to stay on top of market access trends, anticipate how the healthcare landscape is evolving, and provide strategic guidance for your organization. Market Access Macrotrends answers your questions about changes in the access environment related to: • Current and emerging U.S. access trends • Response by stakeholders to market access […]
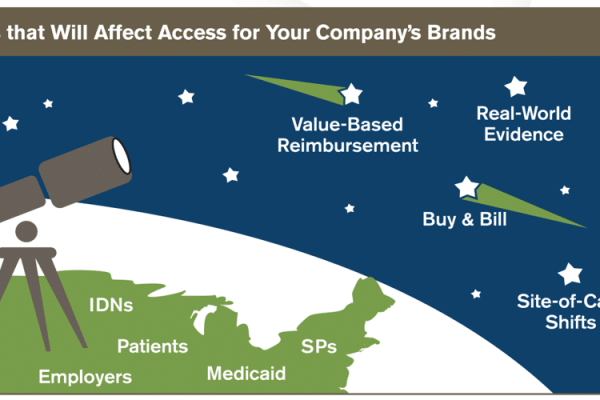
Insurance and Benefit Design
Insurance and Benefits Design provides insight on changes in insurance and benefit design in the next three years so you can anticipate the evolving access opportunities and risks for your company’s brands. Insurance and Benefit Design answers your questions about changes in the access environment related to: • Macrotrends • Benefit design and drug management […]
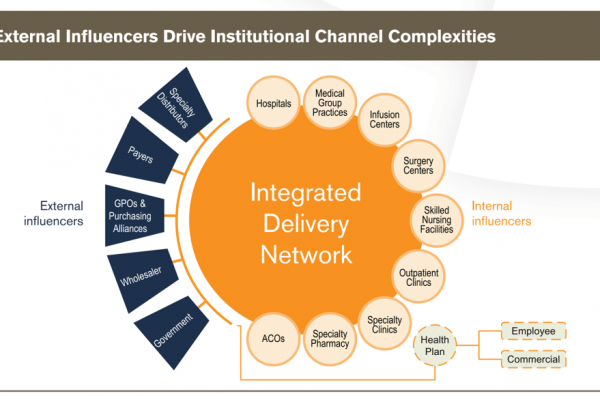
IDN Profiles
IDN Profiles provides insight on the size, structure, and business strategies of the leading IDNs so you can develop business and account plans. IDN Profiles answers your questions so you can develop business and account plans. Who are the leading IDNs? What are the organizational structure, payer mix, and strategic goals of each? How do […]

Macro Trends – Delivery Of Care
Delivery of Care provides insight on changes in delivery of care in the next three years so you can anticipate the evolving access opportunities and risks for your company’s brands. Delivery of Care answers your questions about changes in the access environment related to: Site-of-care shifts Transitions of care Technological advances in care delivery Care coordination […]
Breaking Down the U.S. Payer Segment
Market Access Macrotrends provides insight for you to stay on top of market access trends, anticipate how the healthcare landscape is evolving, and provide strategic guidance for your organization. Market Access Macrotrends answers your questions about changes in the access environment related to: • Current and emerging U.S. access trends • Response by stakeholders to market access […]
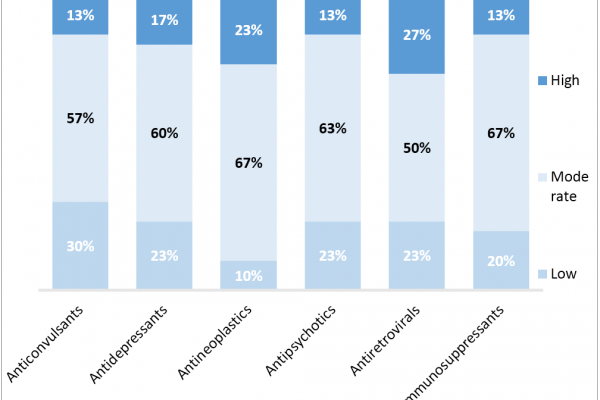
How Could Policy Changes to Protected Classes Impact Part D Access and Contracting?
(Content Updated 5/17) CMS Protected Class Rule Finalized, Slightly Loosened for Biopharma Following CMS’ 2018 proposed rule allowing for new protected class exceptions and a comment period for stakeholders through the beginning of this year, CMS has released a final rule addressing the Medicare Part D Protected Classes. The proposed rule allowed plans to restrict […]
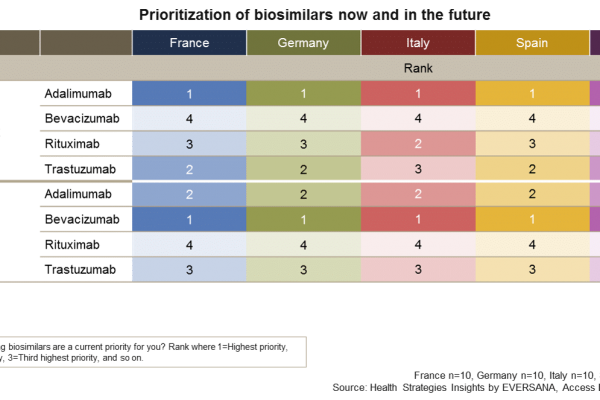
Is your biosimilar product on the payer radar?
Biosimilars have been eagerly awaited in many European countries to realize cost savings from the biologics budget. Gaining insight on how European decision makers are currently prioritizing these biosimilars and how they expect this to shift by 2023 will allow a better understanding of the strategies that may prioritize revenue sources, identifying specific markets and […]

The Brink of the Biosimilar Revolution
Although biosimilars have not had the immediate uptake in the United States that they have had in Europe, health plans expect these products will become a staple of therapy for some diseases by 2020—if the price is right. Since the first biosimilar was approved in the United States in 2015, brand marketers have been bracing […]

