Going Global to Deliver Value
The UK, EU4 and emerging markets across eastern Europe present a major opportunity for U.S. biotechnology companies. However, many such companies decide to license their products to partner companies, assuming that launching in Europe is complex. The result: Often, the product fails to achieve its full value. To be globally competitive, EVERSANA can work with you to assess and adapt your development and commercialization strategies to retain the value of your product.
Europe is Complex, but Not Complicated
Europe is a highly diverse market that provides significant opportunity for any company seeking to globalize its products. With a greater understanding of the region and the individual countries’ nuances, companies can make more informed choices, secure better deal terms with partners and explore their commercialization options. Unlike other markets, regulatory approval, price, reimbursement and access are all intimately linked in Europe. Most countries will not enter reimbursement, access or pricing discussions if a company has not begun the application process for marketing authorization or has one already.
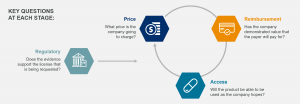
European Commercial Readiness Assessment
Powered by our proprietary, best-in-class price and access platform NAVLIN Price & Access Data, the European Commercial Readiness Assessment helps companies assess their opportunity in Europe. Our team of experts will partner with you to ensure that you have the information you need to maximize your revenue potential.