인사이트
Insights From Our Experts
Articles

The Future of Pharma – A Provocative Perspective
In this episode of AI For Pharma Growth, Dr. Andree Bates is joined by Mike Ryan, Vice President, Europe, EVERSANA. They discuss the future of pharma and the impact of recent advancements in healthcare. The industry…

Single-Arm Data: Turning Limitations into Strengths with Numbers
Randomized controlled trials (RCTs) are at the top of the evidence pyramid because they are designed to be unbiased. Unfortunately, for many medical devices, these types of studies are lacking. This is either because they are…
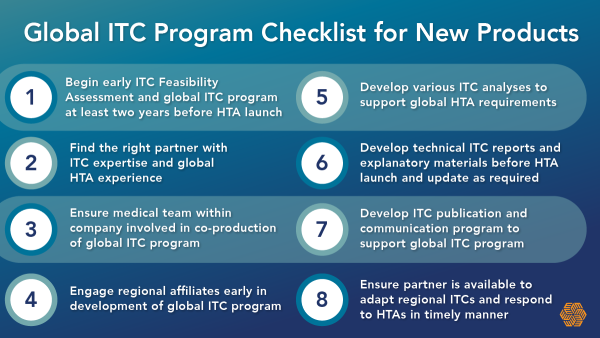
Eight Key Strategic Considerations When Launching a Global Indirect Treatment Comparison Program for New Products
Indirect treatment comparisons (ITCs) refer to a comparison of different healthcare interventions using data from separate studies, in contrast to a direct comparison within randomized controlled trials (RCTs). Indirect treatment comparisons are often used because of…

The State of National Healthcare Policy and Pricing Webinar 2023
2023 is shaping up to be an interesting year for Pharma/Biotech companies and their commercial and government pricing strategies and contracts. There are multiple policies and rules being discussed, already proposed, under review, in litigation, and…

Bringing a CPT Code to Life to Help in the Fight Against Cancer
An interview with Navid Alipour, CEO of CureMatch, and Brian Abraham, Sr. Director, Market Access & Patient Services with EVERSANA On January 1, CureMatch, a leader in precision medicine that has developed a unique analytical platform…

Can Telehealth Accelerate the Uptake of Digital Therapeutics?
Introducing an industry-first, integrated direct-to-patient telehealth solution for digital therapeutics that includes patient engagement, telehealth consultation, prescription issuing and dispensing, and patient support. Fueled by the pandemic, telehealth has moved from an interest to expectation for…

Critical Success Factors for Launching Products with Orphan Drug Designation
The Orphan Drug Act (ODA) was passed in 1983 to financially incentivize pharmaceutical companies to develop drugs for rare diseases or conditions, defined as a disease or condition that affects less than 200,000 people in the…

How Generative AI and Synthetic Content Will Revolutionize Healthcare
The current state of technology is rapidly evolving. Areas such as Artificial Intelligence (AI) have made tremendous strides in generative models and synthetic content, such as ChatGPT, which was recently released. AI has the potential to…
Filling Evidence Gaps Through Consensus: Revisiting the Delphi Technique
With the launch of any medical device, companies and healthcare providers must be thoughtful about answering the following questions: Who will benefit from this device? What problem does it address? How does this device fit into…

Optimizing and Accelerating Membership Management Within the Healthcare Industry
The various complexities and ever-changing nature of the pharmaceutical and health care industries create endless intricate processes, datasets and other components for manufacturers to navigate. Properly maintaining a Customer Master can be one of the most…

