洞察
Integrated Solutions
Rooted in the Patient Experience
Across the Product Life Cycle
Related Articles

Successfully Navigating Self-Commercialisation in Europe
Introduction As discussed in our previous paper, Successful Launches in Europe: Complex, But Not Complicated?,, Europe is one of the world’s largest and most important regions of focus for pharmaceutical and biotechnology manufacturers looking to treat patients and maximise the value of their products. However, the challenges of entering this market, particularly in terms of its complexity, can be intimidating and may deter some companies from launching themselves. Companies may prefer to out-license or sign agreements with distributors, which are the more traditional routes to market. However, these agreements result in less control for the manufacturer regarding all aspects of the launch strategy and decreased long-term company awareness and revenue […]

Successful Launches in Europe: Complex, but Not Complicated?
Europe represents a major pharmaceutical market – it accounts for 22% of the global market, second only to the U.S. in terms of market size and has a large population of over 500 million. Understandably, pharmaceutical and biotechnology companies looking to maximise their revenue consider Europe to be a key focus[1]. Indeed, looking at first-time drug launches by biotech companies, between 2010 and 2020, two-thirds of U.S. launches were followed by launches in other countries[2]. Our paper, “Successful Launches in Europe: Complex but Not Complicated,” discusses the challenges and opportunities associated with launching a pharmaceutical product in Europe. This paper emphasizes the need for pharmaceutical companies to adopt a comprehensive […]

The Future of Pharma – A Provocative Perspective
In this episode of AI For Pharma Growth, Dr. Andree Bates is joined by Mike Ryan, Vice President, Europe, EVERSANA. They discuss the future of pharma and the impact of recent advancements in healthcare. The industry is facing a unique moment, with technology opening up new possibilities for medical interventions and a greater focus on patient outcomes. The combination of artificial intelligence, big data, and shifting customer expectations are driving major changes in healthcare and pharmaceuticals. The industry must be prepared to embrace these changes and take bold steps to succeed in this new landscape. In this episode you will learn: Why healthcare is undergoing rapid change How technological advancements […]

How Specialty Pharmacies Can Elevate Your Channel and Distribution Strategy for HCP-administered Buy & Bill Products
A successful channel and distribution strategy for Buy & Bill (B&B) products should be centered around the partners you sell to and through. That means considering any third-party logistics (3PL) providers, specialty distributors (SD) and specialty pharmacies (SP) who will touch your product between the manufacturing/packaging site and patient administration. Taking a holistic view of the entire product and patient journey will give you the insight you need to make thoughtful and cost-effective decisions as you pull together this critical part of your overall strategy. Procurement and Administration Options for HCP-administered B&B Products With the continuing rise of HCP-administered therapies for multiple disease states in the last five years, it […]

New Medicines, New Access, New Frontiers
How psychedelics, cell therapies and other innovations are driving regulatory reform and new patient access Science is improving exponentially. What’s now possible, through new medicines, and entirely new categories of medicine, could only be dreamt of just a decade ago. Doctors and patients are naturally excited about the oncoming pipeline. But while science improves exponentially, regulators do not. Indeed, many of the checks and balances, as well as stakeholder attitudes, take much longer to reform. The pressure is on: regulatory evolution must be safe and sensible, yet every day’s delay will mean patients are left untreated. In this Impatient Health webinar, EVERSANA’s Mike Ryan, Executive Vice President, Europe, discusses with […]

Impact of Patient Reported Outcome Measures on HTA Decisions for Rare Diseases
As rare diseases become an increasing area of global focus, pharmaceutical manufacturers are taking a closer look at how patient-reported outcomes (PROs) may be used to improve uptake in HTA evaluations. Although “rare” suggests not many people are affected with a condition, in the EU between 6,000 and 8,000 different rare diseases affect an estimated 30 million people collectively, while in United States over 7,000 rare diseases affect more than 30 million people Use of patient-reported outcomes (PRO) in rare disease research and clinical practice offers the potential to improve patient care and outcomes and may also help in critical decision-making, especially for orphan medicines. In our latest white paper, […]

What You Need to Know as the European Union Embarks on Joint Health Technology Assessment (HTA)
Facing the “Fourth Hurdle” Member states of the European Union benefit from a centralised marketing authorisation process for medicinal products. Also, since its implementation in 1993, the European Medicines Agency (EMA) has assured pharmaceutical companies the right to commercialise products that underwent a centralised marketing authorisation in the second largest (and most diverse) single pharma market. However, it remains “the responsibilities of the Member States [to define] their health policy and for the organisation and delivery of health services and medical care. The responsibilities of the Member States shall include the management of health services and medical care and the allocation of the resources assigned to them” (Art. 168, “Treaty […]

How Do We Pay for a Cure? How to Put a Price on Life-changing Treatments
The global cell and gene therapy market is expected to reach $13.8 billion by 2026, expanding at a compound annual growth rate of 12.4%. — Global Cell & Gene Therapy Manufacturing Services Market by Type – Forecast to 2026 Report With over 2,000 ongoing clinical trials in regenerative medicine, patients across the world will soon have even greater access to a large number of life-changing therapies for a variety of diseases. In its 2022 state of the industry report, the Alliance for Regenerative Medicine (ARM) expects at least seven cell and gene therapy approvals in Europe by 2023. A handful of curative therapies have already begun to come to market, […]
How to Ensure Speed and Efficiency When Launching in Europe
Today is an extraordinary time for medical innovation. The pharmaceutical industry is yielding protection against diseases, focusing more on rare and complex disease treatments, and the world is getting better health outcomes. Global life expectancy is more than 70% higher today than it was in 1960 as diseases that were once considered terminal are now treatable. In 2020, the European Union (EU) made a commitment to continue progressing into the future of healthcare with the Pharmaceutical Strategy for Europe. This plan aims to evolve regulatory framework that supports industry and promotes research and technologies that will reach patients’ therapeutic needs while addressing market failures. Specifically, the Strategy will focus on […]

Considering Commercial Success During Clinical Development: Maintaining a Global Perspective
Although commercial success is the ultimate goal of pharmaceutical research and development (R&D), many new treatments do not meet expectations and fail to achieve broad global reimbursement after initial regulatory approval., This causes therapies to underperform across international markets and, more importantly, limits access for patients in need. For example, only 56% of all new drugs approved by the European Medicines Agency (EMA) between March 2000 and March 2018 went on to receive a positive reimbursement recommendation by the National Institute for Health and Care Excellence (NICE) in the United Kingdom (UK)—the country’s central health technology assessment (HTA) body. Several factors contribute to suboptimal global reimbursement, including a bias toward United States (U.S.) market characteristics and the compartmentalization […]

EPP Keynote Address: Global Pricing and Market Access Hurdles for Innovation
Global Pricing and Market Access Hurdles for Innovation In a recent keynote address at the 2021 EPP Life Sciences Pricing Conference, Alan Crowther, General Manager, EVERSANA Pricing and Market Access, discusses 4 hurdles that are serving as obstacles to innovation in global pricing and market access including: Increased pricing pressures Changing launch dynamics that impact patient access More complex global pricing dynamics Rise and changes in HTA During this session, learn how increased global collaboration can serve as a key component in solving these challenges. WATCH NOW:

Pharma Europe 2021 : Panel
A Data and Digital Enabled GTM Strategy for Long-term Business Impact Rohit Sood, Executive Vice President, COMPLETE Commercialization, joined a panel of industry experts to discuss why an aligned and cross-functional go-to-market strategy has never been more important and how to balance strategic priorities in this Pharma 2021 conversation. Watch the recording: Meet with our experts.

Bio-Europe “Collaboration Close-Up”: Exploring the EVERSANA – Shorla Pharma Partnership
Launching in today’s unpredictable market is a journey with new challenges around every corner. While the oncology pipeline is rapidly growing, stringent competition and an evolving provider environment are putting intense pressure on manufacturers to build effective commercialization infrastructures and launch products at unprecedented speeds, which comes at a steep price. In this session, Shorla Pharma, a specialty pharmaceutical company, shares their partnership journey with EVERSANA to bypass the traditional commercialization model that’s too inefficient, costly and cumbersome. Mike and Sharon share their vision of an integrated model that’s streamlined to maximize brand value and improve patient access and outcomes. Panelists include Sharon Cunningham, CEO, Shorla Pharma and Mike Ryan, Executive […]
WEBINAR: Yes EU can! How novel treatments can avoid complexity and commercialize with impact across the European continent
Europe has a population of more than 450 million people, yet most manufacturers consider the U.S. the primary market for launch. In this webinar, EVERSANA’s Mike Ryan, Executive Vice President, Europe, discusses with other industry leaders how the EU’s landscape is changing in order for European states to increasingly become a priority market for commercialization. This conversation takes a closer look at the following topics: How to get past the perceived launch “red tape” in the EU Emerging regulatory, HTA, access, data and commercial routes that will begin to open up this market What a successful post-pandemic launch should look like and determine the steps you need to get there. Investments […]

EPP Studio Live Webinar — Impact of COVID-19 Pandemic on German Healthcare System
Leading up to the recent German elections, the topic of healthcare took center stage for the aging German population. With nearly 90% of the population participating in Statutory Health Insurance (SHI), increased financial pressures have widened the gap between SHI coverage and anticipated expenditures. During this EPP Studio Live Webinar, Douglas Foerster, Senior Vice President of Pricing & Market Access for EVERSANA, and Mathias Flume, Head of Drug Development for KVWL, discuss how various political parties are responding to these challenges and the potential impact on market access and pricing in Germany. WATCH NOW:

EPP Studio Live Webinar — EU Joint Procurement of COVID-19 Vaccines: A Model for Future Market Access in Europe?
The COVID-19 pandemic has changed the way we live and the way we respond to global healthcare crises. During this EPP Studio Live webinar, Douglas Foerster, Senior Vice President of Pricing & Market Access for EVERSANA, shares a brief historical summary of how the European Union negotiated the acquisition of COVID-19 vaccines through an unprecedented, and somewhat controversial, joint procurement process. Mr. Foerster and Professor York Zöllner of the Hamburg University of Applied Sciences discuss the pros and cons of this joint procurement approach as they explore various scenarios under which this process may offer future market access considerations of cost, speed, efficiency and equity. WATCH NOW:

WEBINAR: The New Path to European Pharmaceutical Commercialisation
Global healthcare and life sciences innovation continues to surge at an unprecedented rate. External disrupters such as Brexit, GDPR, new regulatory guidance, such as the Pharmaceutical Strategy for Europe, and the COVID-19 pandemic, are driving pharma and healthcare leaders to reevaluate long-standing strategies. If pharma companies want to remain globally relevant and competitive, they must reassess commercialisation strategies – particularly related to clinical trials, pricing and digital technologies and therapeutics. In this webinar, EVERSANA’s Mike Ryan, Executive Vice President, Europe, and other European commercial leaders from BMS, CSL Behring, Novo Nordisk and Takeda share insight on how to: Choose a commercial strategy that will provide efficient, synchronous, targeted outcomes for patients and providers across all EU countries and the UK Plan […]

EPP Studio Live Webinar — Looking Ahead: Landscape of the Key Price and Market Access Trends in Europe
The global healthcare landscape continues to change at a rapid pace. As a result, new pricing and access trends are emerging across the EU and beyond, driving significant changes in the life sciences industry. During this EPP Studio Live webinar, “Landscape of Key Price and Market Access Trends in Europe,” Alan Crowther, General Manager, Global Products for EVERSANA, shares his insights on how: Increased pricing pressure is being placed on mature portfolios to create room for innovation; The launch of new products is changing, through shifting strategies in launch sequence, market prioritization; Net price transparency and reference pricing are creating competing dynamics in the marketplace; and Pricing regulations are changing, from price control to incentives for supply protection. Watch the full […]

Simplifying EU Distribution to Maximize Cost Efficiency and Speed to Market for Patients and Manufacturers
COVID-19 ignited a spark of innovation in the healthcare industry, forcing global markets to reconsider drug development and commercialization processes. The European Union (EU), specifically, is taking carefully planned steps into a new phase of pharma with recent changes, including the Pharmaceutical Strategy for Europe. But one element of the European pharma industry that remains constant is product distribution through parallel trade. In 2012, parallel trade activity rose by up to 25% in some countries; but distributing a product across territories with diverse regulations, cultures and languages is complex. Now, there is an even more efficient way for manufacturers to distribute treatments in Europe. The answer is one end-to-end commercialization […]

INFOGRAPHIC: What You Need To Know About The Tender Landscape In Europe
In today’s market, manufacturers need real-time pricing insights to remain competitive and develop strategies supported by the right data. PriceRight® by EVERSANA is helping manufacturers manage enterprise and government pricing changes for markets around the world, which is critical as manufacturers launch globally. More specifically, manufacturers launching in Europe must be up to date on regulatory changes, including those related to Brexit and the Pharmaceutical Strategy for Europe. To help deliver key insights to our clients, the PriceRight team surveyed 21 European countries about their price regulation and tendering approach for generic medicines. Talk to one of our pricing experts today. Download some insights from this survey now!

The Secret’s Out: The Impact of Net Price Transparency
Pricing system pressures are increasing for the top four markets in Europe. Price transparency, changes to public health plans and curative therapies are set to disrupt traditional drug pricing. To navigate this ever-shifting landscape, companies must carefully consider product adoption and launch sequencing, and understand the potential impact that net price transparency might have. In this webinar, EVERSANA’s Alan Crowther, General Manager, Global Pricing and Market Access Solutions, and Ed Corbett, Senior Principal, EVERSANA™ CONSULTING, EMEA, discuss: Situation: What’s the current global landscape of net price transparency? Implications: How are the changes going to impact current in-market products and future product launches? Actions: What actions do firms need to take […]

A New Pharmaceutical Strategy for Europe
In late November, the European Commission announced the adoption of a new healthcare plan – the Pharmaceutical Strategy for Europe. This plan aims to strengthen the European Health Union while ensuring affordable patient access across the entire European Union and supporting sustainable innovation for pharmaceutical industries. What’s Changing, and Why? Until now, patient access and health data collection has been hindered by increasing drug development costs and, subsequently, pricing, as well as inconsistent drug availability across the European Union (EU). The onset of COVID-19 further emphasized the need to improve patient access, drug affordability, competitive pricing and overall crises preparedness and response in the EU healthcare system. In particular, the […]

Do Germany’s New Hemophilia Regulations Have Wider-Reaching Implications?
In July 2019, the Drug Safety and Supply Law (GSAV) was passed by Germany’s Higher Chamber of Parliament, bringing about substantial changes to Germany’s health policy. Besides introducing new strategies for biosimilar uptake and cell and gene therapy monitoring, GSAV provides for more stringent control of the distribution of hemophilia products. Download the FULL Pricentric Insights HERE. Learn more about Pricentric ONE and our Global Pricing Solutions! Contact us with your questions and global pricing needs, and an expert will follow up shortly.

Italy Tackles HTA, Pricing and Reimbursement Reforms
In July, new legislation set to overhaul the way pharmaceuticals are priced in Italy was finally implemented, following an announcement that dates all the way back to August 2019. The provision, which was signed by then Ministers Giulia Grillo (Health) and Giovanni Tria (Economy), has rolled out new criteria and methods by which the Italian Medicines Agency (AIFA) determines, via negotiation, the prices of drugs reimbursed by the national health service (SSN). Italy finally rolled out the new policy this year, replacing the “Delibera CIPE” that had governed the Italian pricing and reimbursement of medicines since 2001. Download the FULL Pricentric Insights HERE. Learn more about Pricentric ONE and our […]

eBook – 2020 Stories That Shaped Commercialization
While many industries hit the pause button on their operations this year, the pharmaceutical industry never stopped, propelling new innovations to better serve patients, providers and stakeholders. At EVERSANA, we worked closely with manufacturers to tackle uncertain, complex market dynamics and solve pricing, access, reimbursement, adherence, and distribution challenges, and more. As we look forward to 2021, download EVERSANA’s special edition e-Book, “2020: Stories That Shaped Commercialization,” to recap the trends and stories that drove the industry to new heights this year. eBook Preview: Accelerating Patient Outcomes With Data and Analytics The New Gold Standard of Commercialization The Transformation of Digital Therapeutics Strategic Global Product Launches Pivoting the Patient Experience […]

NEXT GENERATION COMMERCIALIZATION: A CONVERSATION WITH THE INNOVATORS
Sell, out-license or launch internally: these are the three traditional options to commercialize a pharmaceutical product. Selling and out-licensing are common but cause innovators to lose ownership in an investment that takes years to develop. Launching internally requires an average investment of $125MM+ before the product even hits the shelves. Until now, there was no other way to take a product to market. The newest option – EVERSANA™ COMPLETE COMMERCIALIZATION – affords innovators full access to a complete end-to-end commercialization model. In this panel moderated by EVERSANA’s Greg Skalicky and Jim Lang, EVOKE CEO Dave Gonyer and Zosano CEO Steven Lo explain how selecting EVERSANA as their commercialization partner allows […]

How to Get Your Digital Therapeutic Approved & Reimbursed in Europe and the U.S.
Patients, providers, and payers expect healthcare to be more accessible, intuitive, and adaptable to their needs. Barriers that have held back innovation in Digital Medicine and Telemedicine have been blasted apart with the recent relaxing of CMS and HHS requirements, continuing advances within the FDA’s Digital Health program, and additional reimbursement opportunities emerging globally. Get insights on two critical topics: Challenges and imperatives to obtain approval of your Digital Therapeutic Discuss the challenges and imperatives to obtain approval of digital therapeutics in both Europe and the U.S., the continued advances and changes in digital medicine and telemedicine worldwide, and the roadmap to success. Explore the reimbursement opportunities in Europe and […]

The Importance of Digital Strategy in Pharma in the APAC Region
The Asia Pacific region continues to show great progress in digital health as pharmaceutical companies look at digital technologies as a tool to drive access and to start developing their digital strategies. To understand the strides and recent evolution of digital in pharma, our APAC team conducted a survey with top pharmaceutical companies in the region. Amardeep Udeshi, Partner at EVERSANA APAC, outlined the results of the survey and provided insightful recommendations during our Digital Symposium on how the industry is changing. Watch Amardeep’s insights and get his take on the survey results

Improving Patient Outcomes Through Digital Health
Digital applications can work cohesively with therapeutics in the treatment of a broad range of diseases, from behavioral health to chronic conditions. In an interview with Debraj Dasgupta, Head of Strategy & Go-to-Market Planning at Nippon Boehringer Ingelheim Co., Ltd., Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, obtained an insightful perspective on digital transformation in healthcare and how pharmaceutical companies in the APAC region and across the world are leveraging digital technologies. Watch the interview in its entirety

The Digitalization of Healthcare in Asia Pacific
The digitalization of healthcare in Asia Pacific is being utilized to enhance the patient-centered approach and expanding access to health. In a candid conversation with Abhishek Shah, CEO at Wellthy Therapeutics, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, uncovered the current healthcare landscape and how digital strategy in pharma is evolving in the APAC region. Watch this insightful conversation:

The Regulatory Landscape in Digital Health
Despite the advances in digital health and digital therapeutics in recent years, there remains an ambiguity in the digital space and uncertainty surrounding regulation and reimbursement of digital technologies applied to pharma, biopharma and medical device companies. In an insightful conversation that was part of EVERSANA’s Digital Symposium, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine, and Martin Culjat, Sr. Vice President, Regulatory Innovation & Digital Medicine, discussed the use of digital technologies in pharma and the regulations surrounding it. Watch the full conversation:

Access to Technology and the Rise of Digital Health in Asia Pacific
The access to technology, development of telehealth and evolution of digital therapeutics are thriving in Asia Pacific; and pharmaceutical companies are levering the momentum to reach to a broader segment of the patient population. Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, interviewed Anish Shindore, Head of Digital Therapeutics at Sanofi, during our Digital Symposium to understand the critical aspects of the use of digital technologies by pharma companies in the Asia Pacific region. Watch the interview now:

Digital Innovation in Asia Pacific
Digital medicine and digital therapeutics are transforming the healthcare industry by changing the care delivery format, and they have the potential to improve patient adherence and outcomes. In our recent Digital Symposium, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, interviewed David Keene, CEO at IntroSpect Digital Therapeutics & Lead, Digital Therapeutics at ATAI Life Sciences, and they looked at the evolution of digital therapeutics and the shift of the use of digital technologies in healthcare. Furthermore, Ed and David highlighted the trends and innovations in digital health happening in Asia Pacific, pointing out the barriers, opportunities and path forward. Watch the interview:

Outlook: Digital & Pharma in Asia Pacific
Asia Pacific is one of the fastest-growing regions for digital innovation in the pharmaceutical and biotechnology industries. Its promising outlook is mainly driven by necessity, commercial flexibility and a broad talent pool. In a candid conversation broadcasted during the EVERSANA’s Digital Symposium, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine, and Ravi Visweswara, Executive Vice President at EVERSANA APAC, reflected on all the changes and trends in the use of digital technology, providing insightful remarks and an outlook into the future of digital in pharma in Asia Pacific. The two industry experts brought a holistic view of digital health in the pharmaceutical sector and highlighted […]

Leading and Decision-Making in Uncertain Times
By definition, leaders must have followers, who are defined as people who must be shown the way. While this is within most leaders’ grasps when the road ahead is straight and wide and the destination is visible, more is demanded of them when the road splits, narrows and dusk falls. The current crisis presents special challenges to business leaders who have experienced largely smooth sailing for the longest bull market in history. Used to relying on historical data to project the future and to relatively predictable competitive and regulatory events in the world of operations, pharma’s leaders have focused on addressing more strategic challenges, adjusting corporate capability, capacity and culture […]

What kind of clinical data is required for FDA clearance of a Digital Medicine?
What kind of clinical data is required for FDA clearance of a Digital Medicine? It depends. Digital therapeutics and digital medicines that are subject to FDA regulation are considered medical devices. The amount of clinical data required by FDA generally depends on risk. Many moderate risk (class II) medical devices seeking 510(k) clearance are required to perform confirmatory clinical trials to demonstrate that the device is as safe and effective as another legally marketed predicate device. These trials are often single-arm clinical trials, due mainly to challenges of randomizing and blinding medical devices. However, many 510(k) submissions do not require any clinical data. De Novo (usually class II) and PMA […]

What specifically triggers FDA regulation of a digital medicine?
I often meet with Digital Health or Pharmaceutical clients to discuss their commercialization journey, and they ask me the same question. What specifically triggers FDA regulation of a digital medicine? Some of these products require FDA clearance and some do not. Regulatory oversight of digital medicines depends primarily on intended use, which is generally captured in the marketing claims of a product. Software that is intended for diagnosis, treatment, cure, mitigation, or prevention of a disease or condition is considered to be higher risk and is likely to be regulated. Software that indirectly impacts a patient’s health by aiding in their treatment, diagnosis, or triage also typically requires regulatory oversight. […]
Validate and Inform your Account Access Strategies and Tactics
With $250 million in product revenue, our client is the U.S. subsidiary of a European niche biopharmaceutical company looking to build its presence in the U.S. marketplace in anticipation of future product launches. See how the Health Strategies Insights by EVERSANA Team helped the client put an account strategy in place for each key payer account as quickly as possible. Learn more about our Account Access products & capabilities! Contact us with your questions and global pricing needs, and an expert will follow up shortly.
Realizing Value from Digital in the Healthcare Environment [Webinar]
[WEBINAR – 20 MINUTES] The advancements of Digital Therapies are promising but commercial success is not always guaranteed. This presentation explores the pitfalls and challenges of selling products into the uniquely complicated and regulated healthcare market. It will lay the groundwork for digital companies to have successful commercial strategies and be able to: Answer questions like: What is my commercial model? What is my pricing model? How are payers thinking about my disease? Who pays today? Will I get coverage? How does a doctor’s prescription turn into a patient’s download and use? What answers do I need now vs. later? Download the slides OR view the 20 minute webinar.

The New Roaring 20s: How Outsourced Commercialization is the Revolution We Need
The “Great Gatsby Era” was an exciting time of new prosperity, infinite creativity and dramatic social change. Long-standing social norms and traditions gave way to the “mass culture” and consumerism that modernized American society. “What will they think of next?” was a popular expression that defined the era. On the 100-year anniversary of this revolutionary decade, the pharma industry should take note: breaking from tradition is the catalyst for real, long-lasting transformation. Innovative therapies require innovative thinking. In just the past 10 years, we’ve seen both clinical and manufacturing outsourced to single partners who successfully reduced costs, minimized risk, and maintained product integrity. Commercialization, on the other hand, is lagging […]

Improving the Impact of an Oncology Patient Access Program
Our client, a specialty pharmaceutical company focused on the research and development of new therapies in two high-cost complex drug categories, sought to benchmark its patient assistance program against those of its competitors in order to improve relationships within target oncologists’ offices. The Challenge The client needed to assess the image of its patient assistance program among oncologist, practice manager, and specialty pharmacy partners. Specifically, our client wanted to: Benchmark customer perceptions of its patient assistance program versus those of its competitors, Assess the impact of the program on patients and physicians, Identify unmet needs of patient services program offerings, and Identify opportunities to enhance and improve patient services offerings. […]

Strategies to Ensure Market Success of a Rare Disease Product Launch
Small patient populations, complex administration, high costs of therapies, and government policy interventions are just a few obstacles in rare disease. EVERSANA’s Managing Director, Bill O’Bryon, and Senior Director, Diann Johnson, outline key insights to consider in your launch strategy: Market dynamics impacting rare disease therapies The entire rare community: patients, caregivers, providers & advocates Payer landscape and engagement Patient experience Stakeholder relationships Watch the Webinar: Strategies to Ensure Market Success of a Rare Disease Product Launch

After FDA Approval, Can Gene Therapies Achieve Marketing Success?
Without a doubt, gene therapy is transforming healthcare by revolutionizing patient care from conventional treatment models to curative therapy models. There are more than 7,000 distinct types of rare and genetic diseases and 400+ million individuals suffering from a rare disease. With a market that is fragmented, there is a need for an innovative, end-to-end commercial solution to support these new therapies and lead the pharmaceutical product lifecycle from clinical trial recruitment though commercialization. In this point of view published in the special November/December 2020 Preview issue of PharmaVOICE, Greg Skalicky, Chief Revenue Officer, discusses what is needed for gene therapies to succeed after FDA approval. To read the article […]

Global Biosimilar Competitor Assessment – Who Wins in Biosimilar Access and Why?
The introduction of biosimilar products has created interesting changes in payer behavior across the global markets. For both innovator and biosimilar companies, it has been an effort to understand the demands of payers and succeed in what looked like a ‘winner-takes-all’ opportunity. As the first round of biosimilar introductions has passed, there are some interesting findings across the western pharmaceutical markets. Biosimilar Competition Reveals Unique Buyer Types Among European and US Stakeholders EVERSANA identified two unique buyer types when conducting a segmentation analysis based on the reaction of EU and US customers to biosimilar competition and company performance. Economic buyers (e.g., France, Germany, and select payers of the US) treat […]

How do we pay for a cure?
Setting drug prices is a high-stakes endeavor, and that is especially true for the latest round of gene and curative products coming to market in Europe. Drugmakers need to consider several factors that can help them set their product prices appropriately, including the risk of recurrence/relapse, differences in efficacy among cures, and comparators, including full lifecycle costs of a disease and the burden of health economic costs on budgets. They also need to design innovative payment models that will be attractive to health systems facing increased budgetary pressures as more gene and curative products enter the market. This includes outcomes- and performance-based schemes, which have gained momentum across Europe. However, […]

Innovative Therapies Call for an Integrated Drug Safety and Compliance Model
In this article, Herbert Lee, PharmD, Senior Vice President, Medical Communications and Pharmacovigilance, makes the case for an integrated approach to ensuring the safe and effective use of medications by patients. From emerging therapies to innovative technologies, the healthcare industry is changing – demanding that we do business differently. Working in one of the most regulated industries, we need to understand the complexities of developing products/services that meet patient need and help improve the health and safety of the public. Safety and compliance are important areas of focus in every industry I can think of and especially in life sciences. As the global environment evolves, so must our models for […]

Navigating Channel Distribution in a New (and more complex) Biopharma World
Every twist and turn in the bench-to-bedside commercialization journey introduces increasing regulatory complexity, product-specific special handling requirements, and evolving industry guidelines. With more than 300 client case studies to draw from, we’ve found three opportunities (or pitfalls) that manufacturers must address in their channel distribution strategy: How quickly can you get your product into your patients’ hands? What will happen tomorrow if demand doubles tomorrow? Are you prepared for regulatory scrutiny? Download “Navigating Channel Distribution in a New (and more complex) Biopharma World” to learn how to ensure economic success and operational efficiency.

Are We Transforming In The Right Way?
Why Product Launches Can’t Be Distracted By Empty Promises. We can all agree that innovations in therapeutic development have advanced beyond traditional product launch strategies and service models. In every step of the product lifecycle, we see pockets of transformation. The problem is exactly that – “pockets” of transformation. Your product launch strategy has to achieve the following: Value the voice of the patient Protect your revenue Reach all stakeholders now Prepare for optimal distribution Achieve broad and quality access by reaching all stakeholders In this point of view published in the September issue of Pharmaceutical Executive, Greg Skalicky, Chief Revenue Officer, argues that traditional service silos stand in the way […]

Gene Therapy for Rare Disorders
With only three approved products in the marketplace, how do we build a regenerative medicine ecosystem that delivers more value to patient’s faster?
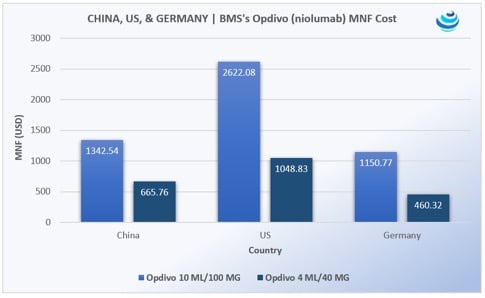
‘Tis the season for pharma in China, as the government expedites uptake of new urgently needed drugs
PRICENTRIC BRIEF: Universal health insurance coverage for 1.3 billion people means China must increase uptake of “clinically urgently needed new drugs” already approved in the US, EU, and Japan Mostly oncology products are being imported, along with HIV/AIDS medicines Pharma is agreeing to steep discounts to be reimbursed on China’s national drug lists THE DETAILS In 2016, China announced that it would be merging the New Rural Cooperative Medical Scheme (NRCMS) and Urban Employee Basic Medical Insurance (UEBMI) to form an integrated system of basic medical insurance for both rural and urban residents, as part of the 2009 Health Care Reform Plan, which seeks universal coverage for 1.3 billion people […]
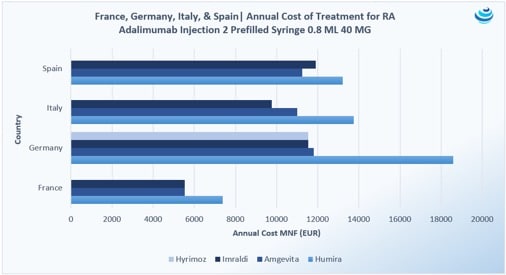
“Who wants the biggest slice of the biosimilar pie?”: The Humira biosimilar wave in Europe
PRICENTRIC BRIEF: Biosimilar competition in Europe has brought about discounts to AbbVie’s blockbuster immunosuppressant drug Humira upwards of 80% during tendering Overall, biosimilar uptake has increased in Europe because biologic “copycats” are cheaper, but full faith in these products is still required from physicians and patients In the US, Coherus struck preemptively with a 33% discount to its Neulasta biosimilar to ensure market uptake THE DETAILS Health authorities the world over seek serious discounts when biosimilar competition enters the market, in many cases mandating discounts and price ceilings to both the originator drug and biosimilars or engaging in tendering practices that allow originator drugs and biosimilars to bid at a […]

Biosimilar Pricing in Europe
This report titled Biosimilar Pricing in Europe is published by Pricentric, by EVERSANA. It examines the pricing and pricing trends of biosimilar drugs in the US and EU5. It particularly looks at the pricing of Infliximab and its effect in the markets.

The Brink of the Biosimilar Revolution
Although biosimilars have not had the immediate uptake in the United States that they have had in Europe, health plans expect these products will become a staple of therapy for some diseases by 2020—if the price is right. Since the first biosimilar was approved in the United States in 2015, brand marketers have been bracing for the impact, given Europe’s fairly broad adoption of these products. But so far, biosimilars have not had a seismic effect on the U.S. market. This has been disappointing for both medical and pharmacy directors in U.S. health plans, who are eager to reap potential cost savings from biosimilars. This white paper examines why the […]

