最新观点
Insights From Our Experts
Articles

BLOG: The ABCDs of Medicare Drug Coverage
The Medicare benefit is divided into four parts, each providing a unique drug benefit. Together, the four parts provide medication coverage – from oral medications taken at home to IV treatments given in a physician’s office…

Webinar: CMS Final Rule on Cost-Sharing Assistance
The CMS final rule on drug reimbursement addresses how co-pay coupons and vouchers are being exempted from deductibles by PBMs. The rule seems to ensure that the full value of manufacturers’ coupon and voucher programs accrue…

Change Is Happening in the EU: What Pharma Companies Need to Know About the European Union’s New Pharmaceutical Strategy
For the first time, the European Union is undergoing a major overhaul of its pharmaceutical industry with the new Pharmaceutical Strategy for Europe, which was launched in late 2020. Through this strategy, the European Union (EU) is making…

News Alert: MCIT Implementation Delayed
On Friday, March 12, the Centers for Medicare and Medicaid Services (CMS) issued an Interim Final Rule with Comment (IFC) delaying the effective date of the Medicare Coverage of Innovative Technologies (MCIT) initiative from March 15…

BLOG: Not So Rare… The True Impact of Rare Diseases
Rare disease treatments seem to be everywhere today. This is true despite the fact that to be considered a rare disease, fewer than 200,000 patients are affected — and for ultra-rare, fewer than 7,000 patients are affected. This definition was created by Congress in the Orphan Drug Act of 1983. Rare diseases became known as orphan…

Global Shifts in Legislative Regulation Are Forcing Pharma Back to the Drawing Board : February 2021
In only a short time this year, the pharmaceutical industry is witnessing new regulations and legislative changes going into effect globally – and the growing pains are setting in. Between the new U.S. administration and E.U. Pharmaceutical Strategy, governmental entities…
BLOG: Population Health Leadership
“Population health” is the result of pushing health systems to be clinically and financially responsible for their communities. This is a significant shift from simply being focused on the volume of services delivered within the hospital. The focus on population health represents a movement for pharma from…
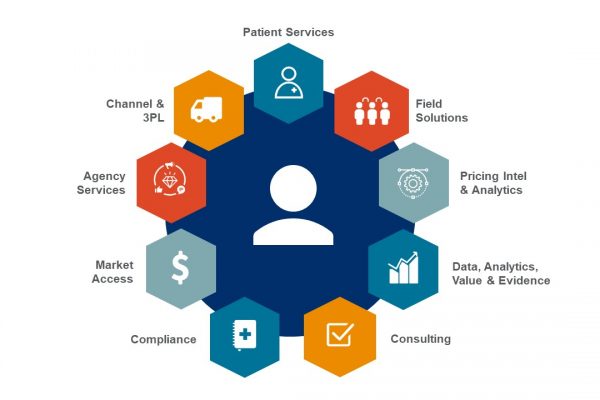
Reinventing Launch: The Gold Standard Of Drug Commercialization
In a world that is rapidly changing, we must evolve beyond traditional strategies to create true impact for patients. EVERSANA's complete end-to-end commercialization model enables manufacturers to bring their drug to market at a fraction of…

The Crush: How Covid-19 Is Impacting Mature Brand Revenue & Long-Term Value
The impact of COVID is reverberating across all aspects of society and business. In the healthcare industry, hundreds of thousands of patients are not getting proper access to and utilization of therapies that can improve their…

Reimbursement May Be on the Way for Remote Therapeutic Monitoring
The American Medical Association (AMA) manages the procedure code set that physicians and other healthcare professionals use to identify the services for which they bill. These codes are commonly known as the CPT® code set, which…

