
NAVLIN Price & Access Data
NAVLIN Price & Access Data is a powerful competitor intelligence tool that provides near real-time updates to drug price (list / net), reimbursement, tender and cost of treatment information at the indication level.
Our team of experts track real time pricing changes across 87+ markets and 200+ therapeutic areas and develop county profiles to inform global pricing and market access strategies for our pharma clients.
Articles by NAVLIN Price & Access Data
Global Regulations Push Accelerated Approvals, Access and Treatment Distribution: June 2021
Improving patient access and treatment affordability is quickly becoming a global priority reflected in current regulatory actions. This month, the U.S. FDA approved Aduhelm (aducanumab) for Alzheimer’s through an Accelerated Approval pathway, which will open treatment opportunities for patients. Meanwhile, the world continues to push on in the fight against COVID: The EU is proposing a multilateral trade action plan to promote broad production of and fair access to COVID-19 medications, and […]

INFOGRAPHIC: What You Need To Know About The Tender Landscape In Europe
In today’s market, manufacturers need real-time pricing insights to remain competitive and develop strategies supported by the right data. PriceRight® by EVERSANA is helping manufacturers manage enterprise and government pricing changes for markets around the world, which is critical as manufacturers launch globally. More specifically, manufacturers launching in Europe must be up to date on regulatory changes, including those related to Brexit […]

Global Pharma Heads Into Uncharted Territories in Pricing and Regulation: May 2021
Policymakers around the world continue to face unprecedented decisions about pharmaceuticals and the healthcare industry. With rapid industry innovation comes new challenges, unanswered questions and intense debates on pricing and regulation. This month, the industry saw progression in rare disease education for policymakers, a spotlight on drug price transparency bills and pricing penalties, advancements in digital therapeutics, development in global HTA use […]

Beyond the New Normal: Reshaping Life Sciences Pricing Strategies in a Complex Global Scenario
This Life Sciences Dynamic Talks session takes a close look at the future of life sciences pricing strategies in the “new normal.” From non-traditional blocs to Brexit and the rise of net price transparency, a number of global trends exacerbated by COVID-19 are increasingly affecting pricing for pharma. In this session, EVERSANA’s Alan Crowther identifies […]

Pharma’s Push and Pull: Products Approved Amid Government Pushes for Regulation and Pricing Change: April 2021
Global healthcare and life sciences innovation continues to surge at an unprecedented rate, and government entities worldwide are trying to keep up. Price transparency and treatment affordability remain at the top of the list for legislative concern. For instance, United States Senator Jeff Merkley (D-OR) and Representative Peter Welch (D-VT) have introduced the End Price Gouging for Medications Act, which would ensure Americans […]

The Secret’s Out: The Impact of Net Price Transparency
Pricing system pressures are increasing for the top four markets in Europe. Price transparency, changes to public health plans and curative therapies are set to disrupt traditional drug pricing. To navigate this ever-shifting landscape, companies must carefully consider product adoption and launch sequencing, and understand the potential impact that net price transparency might have. In […]

Global Shifts in Legislative Regulation Are Forcing Pharma Back to the Drawing Board : February 2021
In only a short time this year, the pharmaceutical industry is witnessing new regulations and legislative changes going into effect globally – and the growing pains are setting in. Between the new U.S. administration and E.U. Pharmaceutical Strategy, governmental entities are starting to set new pricing standards and expectations. The rise of net price transparency, U.S. reference pricing and the prioritization of […]

A Time for Transition in Pharma: January 2021
So far, 2021 is proving to be a time for international transition in pharma. In the latest Pricentric INSIGHTS newsletter, we’re taking a closer look at new legislation and regulatory actions happening around the globe to help you prepare for what’s coming next this year. Here are the top stories from January: Trump’s MFN Rule […]

A Rundown of 2020 Trends in Pharma
As we begin 2021 and vaccines roll out across the globe, it seems that the world is hopeful and eager to move forward from 2020. The COVID-19 pandemic impacted almost every aspect of our lives, wreaking havoc on numerous industries and forcing global companies to re-evaluate their future. But one industry that was consistently in the spotlight was the pharmaceutical industry. The world watched as pharma players […]

How Does the Biden Administration Want to ‘Take on Pharma?’
Throughout his campaign, Biden has promised to, once in office, expand access to health care through the “Biden Plan,” an evolution of former President Barack Obama’s Affordable Care Act, dubbed “Obamacare,” while “taking on big pharma” and reining in “runaway” drug prices. As with Trump, Biden seeks to leverage the negotiating power of Medicare when […]

Do Germany’s New Hemophilia Regulations Have Wider-Reaching Implications?
In July 2019, the Drug Safety and Supply Law (GSAV) was passed by Germany’s Higher Chamber of Parliament, bringing about substantial changes to Germany’s health policy. Besides introducing new strategies for biosimilar uptake and cell and gene therapy monitoring, GSAV provides for more stringent control of the distribution of hemophilia products. Download the FULL Pricentric […]

Italy Tackles HTA, Pricing and Reimbursement Reforms
In July, new legislation set to overhaul the way pharmaceuticals are priced in Italy was finally implemented, following an announcement that dates all the way back to August 2019. The provision, which was signed by then Ministers Giulia Grillo (Health) and Giovanni Tria (Economy), has rolled out new criteria and methods by which the Italian […]

At the COVID-19 Finish Line, How Do We Price The Winning Vaccine?
As of August 21, 2020, there were more than 165 potential vaccine candidates in development for the novel coronavirus. While some are still in preclinical stages, a handful are quickly making their way toward regulatory approval, meaning that pricing is starting to become an urgent reality. The first safety trials in humans started as early […]

Is BeNeLuxA Equipped for a Zolgensma Assessment?
The BeNeLuxA initiative is set to take on Zolgensma (onasemnogene abeparvovec), an innovative gene therapy for children under two years old with spinal muscular atrophy (SMA), which was granted conditional approval for use in Europe in May. Belgium, Ireland, and the Netherlands will undertake a joint health technology assessment (HTA) of Zolgensma as part of […]
Pricentric ONE Newsletter | June 2020
Good news, this month’s issue of the Pricentric Newsletter is now available for download. Download the Newsletter!

What Canada’s IRP Changes Could Mean for the Industry
Last August, Canada’s Patented Medicine Prices Review Board (PMPRB) announced a set of amendments for its regulatory measures, in a bid to strengthen and modernize the country’s pricing framework for patented drugs. Following the initial proposal of the alterations, the PMPRB released its new draft guidelines in November 2019 and launched a 60-day consultation period […]
Pricentric ONE Newsletter | May 2020
Good news, this month’s issue of the Pricentric Newsletter is now available for download. Download the Newsletter!

Pricentric ONE Newsletter | April 2020
Good news, this month’s issue of the Pricentric Newsletter is now available for download. Download the Newsletter!

Is ICER Increasing Influence in the U.S. Over Time?
Recent high-profile product launches have reignited the debate on healthcare costs in the US. The Institute for Clinical and Economic Review (ICER) is an independent research organization that determines value-based price benchmarks. In a recent study, Dr. Stino, Product Manager, examined recent high-profile launches to understand the interaction between ICER value-based price benchmarks and actual […]

How do we pay for a cure?
Setting drug prices is a high-stakes endeavor, and that is especially true for the latest round of gene and curative products coming to market in Europe. Drugmakers need to consider several factors that can help them set their product prices appropriately, including the risk of recurrence/relapse, differences in efficacy among cures, and comparators, including full […]
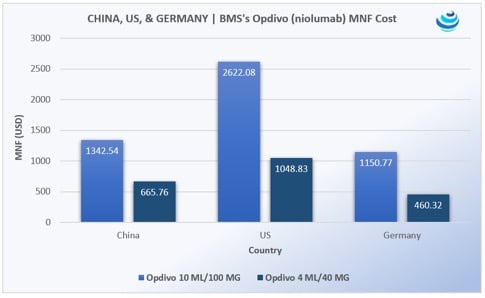
‘Tis the season for pharma in China, as the government expedites uptake of new urgently needed drugs
PRICENTRIC BRIEF: Universal health insurance coverage for 1.3 billion people means China must increase uptake of “clinically urgently needed new drugs” already approved in the US, EU, and Japan Mostly oncology products are being imported, along with HIV/AIDS medicines Pharma is agreeing to steep discounts to be reimbursed on China’s national drug lists THE DETAILS […]
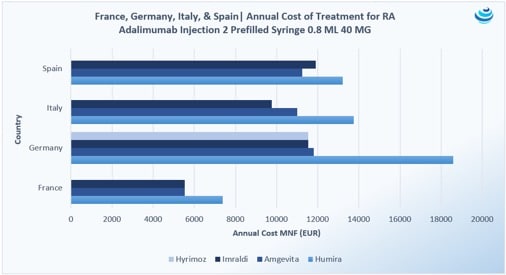
“Who wants the biggest slice of the biosimilar pie?”: The Humira biosimilar wave in Europe
PRICENTRIC BRIEF: Biosimilar competition in Europe has brought about discounts to AbbVie’s blockbuster immunosuppressant drug Humira upwards of 80% during tendering Overall, biosimilar uptake has increased in Europe because biologic “copycats” are cheaper, but full faith in these products is still required from physicians and patients In the US, Coherus struck preemptively with a 33% […]

Biosimilar Pricing in Europe
This report titled Biosimilar Pricing in Europe is published by Pricentric, by EVERSANA. It examines the pricing and pricing trends of biosimilar drugs in the US and EU5. It particularly looks at the pricing of Infliximab and its effect in the markets.

