The First & Only Go-To Complete Commercialization Expert
Manufacturers spend >$125MM over three years leading up to launch, yet 66% of drugs don’t meet launch expectations. An unpredictable landscape, coupled with inevitable industry pressures, is forcing manufacturers to seek a more complete commercialization approach with less risk and more value.
In 2018, EVERSANA, a leading provider of commercial services to the life science industry, implemented a strategy to offer a complete, full-scale, customized model for product commercialization. Our end-to-end commercialization engine, officially referred to as EVERSANA™ COMPLETE COMMERCIALIZATION, gives manufacturers full access to launch strategy, execution and long-term outsourced services (including distribution, field support and patient hub services) through a contracted, multi-year model.
EVERSANA™ COMPLETE COMMERCIALIZATION brings drugs to market with an end-to-end commercialization engine powered by organic connectivity and synergy.
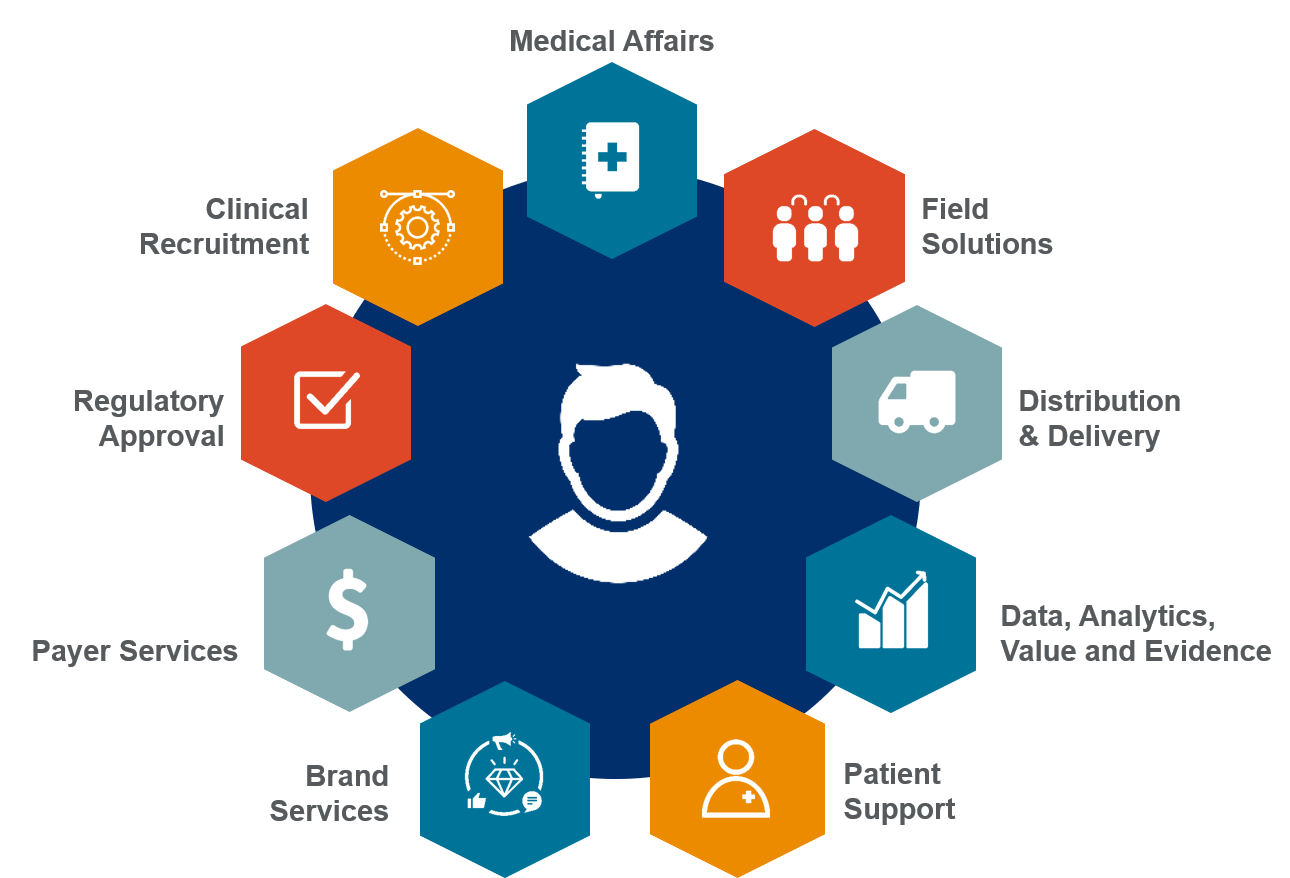
Download Greg Skalicky latest’s article to learn how our complete end-to-end commercialization model enables manufacturers to bring their drug to market at a fraction of the cost of “going it alone” or partnering with another pharmaceutical company.
Author Team

EVERSANA employs a team of over 6000 professionals across 20+ locations around the world. From industry-leading patient service and adherence support to global pricing and revenue management, our team informs the strategies that matter…

