洞察
Insights From Our Experts
Articles

Successful Launches in Europe: Complex, but Not Complicated?
Europe represents a major pharmaceutical market – it accounts for 22% of the global market, second only to the U.S. in terms of market size and has a large population of over 500 million. Understandably, pharmaceutical…

Accelerated Approval Pathways: Manufacturers Left to Grapple With Risk Versus Reward
Oncology and hematology are extremely complex, crowded and chaotic therapeutic areas. With 30% of drug approvals by the FDA occurring in the oncology and hematology space, manufacturers of these high-science brands are often faced with unprecedented…

Understanding the Inflation Reduction Act: Inflation Rebates
Prescription drug manufacturers routinely implement price increases for a variety of reasons that support their business. With the passage of the Inflation Reduction Act late in 2022, Congress added a twist to these price increases. One…

The Pitfalls of Government Price Setting: Value Assessments in the U.S. vs. Germany
As the U.S. considers implementation of the drug pricing provisions of the Inflation Reduction Act (IRA), which includes price negotiation for a limited subset of drugs, it is important to understand the nuances of a government…

Filling the Missing Spaces in Your Network Meta-Analysis: The Role of Surrogate Models
Network Meta-Analysis Network meta-analysis is a statistical method that combines data from multiple randomized controlled trials to compare the relative effectiveness of different interventions (Dias et al. 2011). This method allows researchers to synthesize the results of…

The Future of Pharma – A Provocative Perspective
In this episode of AI For Pharma Growth, Dr. Andree Bates is joined by Mike Ryan, Vice President, Europe, EVERSANA. They discuss the future of pharma and the impact of recent advancements in healthcare. The industry…

Single-Arm Data: Turning Limitations into Strengths with Numbers
Randomized controlled trials (RCTs) are at the top of the evidence pyramid because they are designed to be unbiased. Unfortunately, for many medical devices, these types of studies are lacking. This is either because they are…
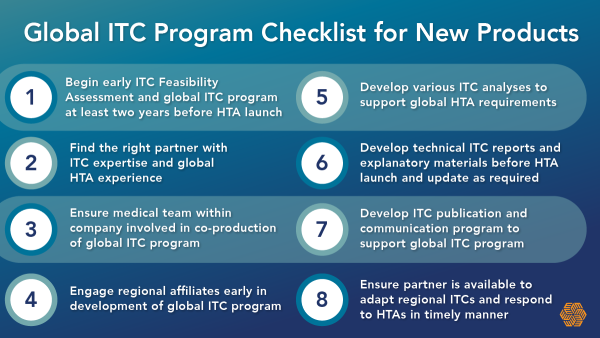
Eight Key Strategic Considerations When Launching a Global Indirect Treatment Comparison Program for New Products
Indirect treatment comparisons (ITCs) refer to a comparison of different healthcare interventions using data from separate studies, in contrast to a direct comparison within randomized controlled trials (RCTs). Indirect treatment comparisons are often used because of…

The State of National Healthcare Policy and Pricing Webinar 2023
2023 is shaping up to be an interesting year for Pharma/Biotech companies and their commercial and government pricing strategies and contracts. There are multiple policies and rules being discussed, already proposed, under review, in litigation, and…

Bringing a CPT Code to Life to Help in the Fight Against Cancer
An interview with Navid Alipour, CEO of CureMatch, and Brian Abraham, Sr. Director, Market Access & Patient Services with EVERSANA On January 1, CureMatch, a leader in precision medicine that has developed a unique analytical platform…

