洞察
Insights From Our Experts
Articles

Will the U.S. adopt global reference pricing?
This article was written for Med Ad News Magazine There are many things that Americans get from outside our U.S. borders. Our top imports include oil, machines, and cars; our iTunes and Spotify accounts are stocked…

Pathways for Paying for Rare Disease Treatments
This article was written for the Journal of Clinical Pathways Determining how to pay for the treatment of uncommon yet serious diseases is an important consideration in terms of sustainability and patient access. Novel and expensive…
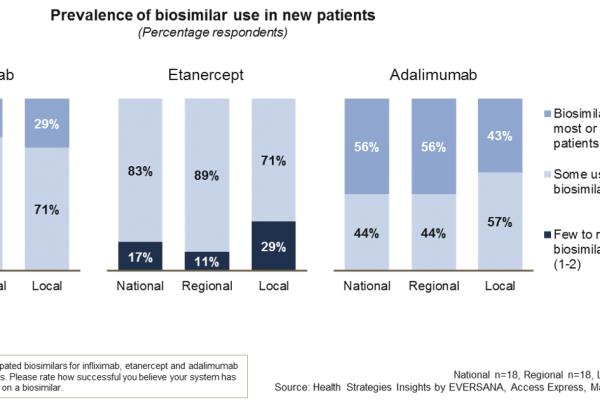
New start versus switch patients for biosimilars?
Over the last several years, European market demand for biosimilars has accelerated with the entry of biosimilars for many reference biologics, including high-value molecules such as adalimumab, infliximab and etanercept. However, there have been reported variations…
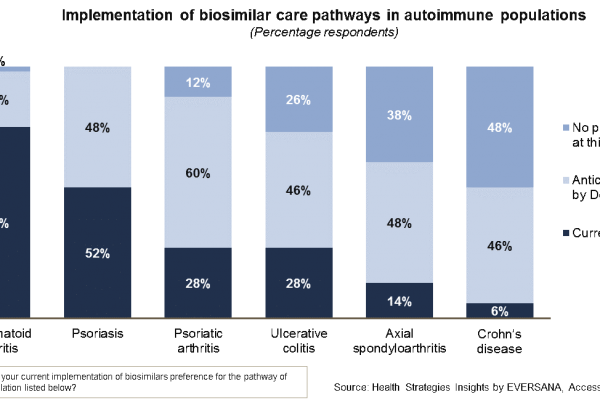
Care pathways—do you know where your biosimilars are used?
Biological therapies are often very expensive, putting pressure on healthcare budgets that are already restricted and potentially resulting in a decrease in patient access to treatment, in Europe biosimilar versions have been eagerly awaited in many…
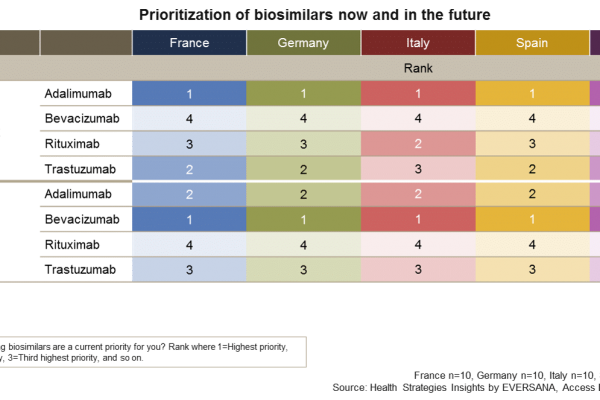
Is your biosimilar product on the payer radar?
Biosimilars have been eagerly awaited in many European countries to realize cost savings from the biologics budget. Gaining insight on how European decision makers are currently prioritizing these biosimilars and how they expect this to shift…
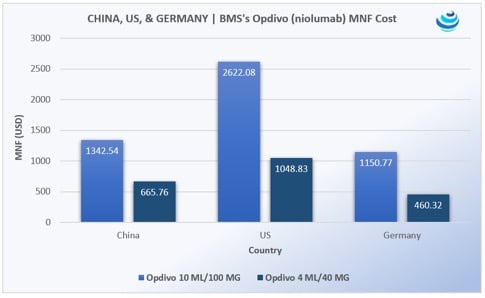
‘Tis the season for pharma in China, as the government expedites uptake of new urgently needed drugs
PRICENTRIC BRIEF: Universal health insurance coverage for 1.3 billion people means China must increase uptake of “clinically urgently needed new drugs” already approved in the US, EU, and Japan Mostly oncology products are being imported, along…
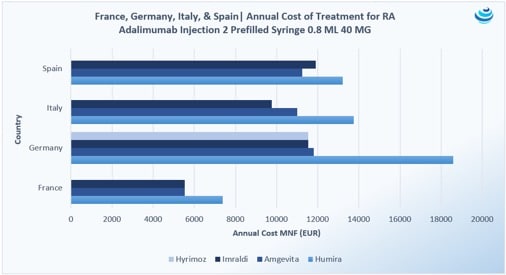
“Who wants the biggest slice of the biosimilar pie?”: The Humira biosimilar wave in Europe
PRICENTRIC BRIEF: Biosimilar competition in Europe has brought about discounts to AbbVie’s blockbuster immunosuppressant drug Humira upwards of 80% during tendering Overall, biosimilar uptake has increased in Europe because biologic “copycats” are cheaper, but full faith…

Medicare & Medicaid: A Rollback on Rebates
In January 2019, the Department of Health and Human Services (HHS) put forth a proposed rule to repeal the safe harbor status for Medicare and Medicaid drug rebates under the Anti-Kickback statute. This idea was first…

Product Master Data Management
This white paper shares considerations, common pitfalls and key takeaways for manufacturers participating in Medicaid. Recent market and enforcement trends in healthcare further exemplify the transition from a volume to value-based marketplace, as well as the complexities…

Precision Medicine Meets Precision Patient Support
In the value-driven healthcare environment, orphan drug manufacturers are becoming increasingly aware that improving adherence requires a new approach to patient understanding and support. Take for example the required lifestyle, nutritional or physical modifications often necessary…

