Over the last several years, European market demand for biosimilars has accelerated with the entry of biosimilars for many reference biologics, including high-value molecules such as adalimumab, infliximab and etanercept. However, there have been reported variations between products and at the different levels of the healthcare system across markets.
In order to investigate how the use of biosimilars may differ, we performed a double-blinded survey that included 50 current or recent decision makers/influencers of healthcare policy across the top 5 EU countries. To participate, each respondent was required to hold or have held a position in a national, regional, or local decision-making entity.
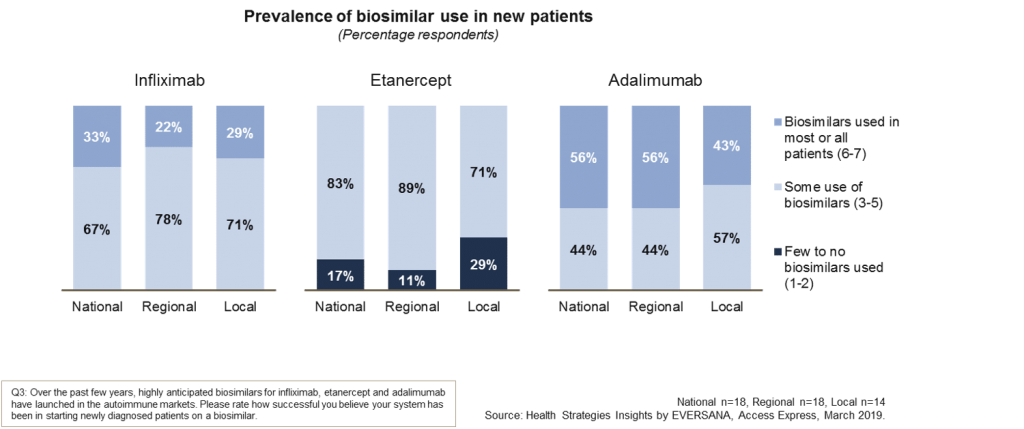
The graph below shows the answers split out by national decision makers (n=18), regional decision makers (n=18) and local decision makers (n=14) across the three products for use in new patients.
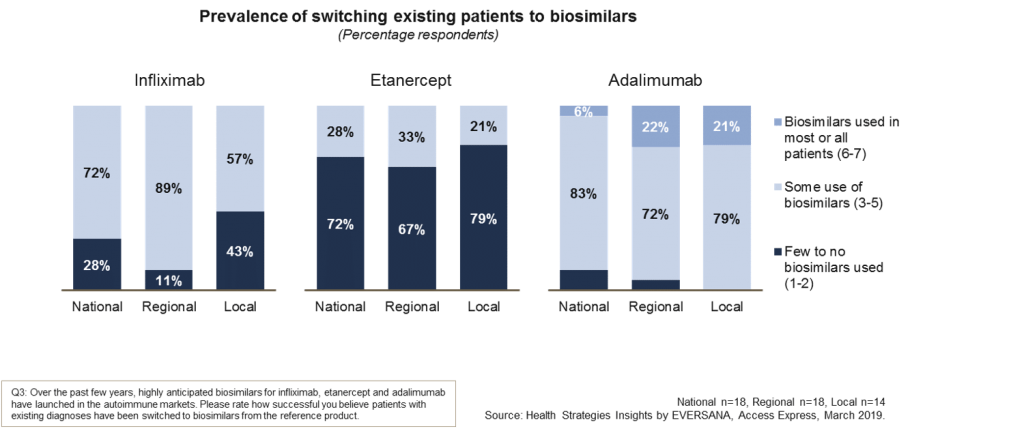
By contrast, the following graph shows the same breakout but this time across the use of the products in patients who are switched either from the originator or another biosimilar.
The responses to the survey illustrate the differences when it comes to biosimilar adoption in newly diagnosed patients vs those with existing diagnoses as well as between different biosimilar entities.
Payers indicate that biosimilar use in new patients is widespread for both infliximab and adalimumab with more than half of new cases being able to utilize the biosimilar in most or all patients. There are, however, still some challenges to the use of the etanercept biosimilar in new patients and this may be due to the different formulations available.
On the other hand, payers report less success in switching existing patients to biosimilars across all three entities with the largest proportion of low use reported again for the etanercept biosimilar but will a substantial proportion of low use (20%) for infliximab.
These results indicate that there is still some resistance to the use of biosimilars in patients who are existing users of biologics and the resulting opportunity to increase access in these patients. Market access teams need to appreciate that increased efforts may be needed to convince payers, physicians and patients to switch and prepare real world evidence accordingly.
To get more insight on our recent biosimilar study, click here for free access to our biosimilar e-book series, EU Decision Maker Perceptions of Biosimilars.

