最新观点
Integrated Solutions
Rooted in the Patient Experience
Across the Product Life Cycle
Related Articles

Establishing the Pricing and Value Proposition of AI/MedTech Solutions in Healthcare
Authors: Prachi Singh, Senior Consultant; Robin Arnold, EVP, APAC Consulting Unlock the future of healthcare with our white paper, “Establishing the Pricing and Value Proposition of AI/MedTech Solutions in Healthcare.” Discover the essential components of pricing AI/MedTech solutions and crafting compelling value propositions that resonate with stakeholders, including payers, patients, and physicians. Explore real-world examples, […]

Measuring Willingness to Pay: The Next Access Frontier
EVERSANA’s APAC Team Authors: Robin Arnold, Swapnil Waichale, Lakshmi Pragna Kalavacharla, Utkarsh Sahu Patient access to expensive healthcare poses a significant challenge in the pharmaceutical industry. As the cost of developing innovative medicines rises, the demand for accessibility as a basic human right increases. This POV explores the evolution of Patient Access Programs (PAPs) from […]

Navigating the Patient Journey: Understanding, Challenges, and Solutions in Patient Journey Mapping
EVERSANA’s APAC Authors: Ankit Kulshrestha, Robin Arnold This POV explores the intricacies of Patient Journey Mapping, detailing its significance, challenges, and solutions. Patient journey mapping is a comprehensive discipline that traces a patient’s entire healthcare journey, from initial symptoms to treatment and beyond. Understanding this journey is imperative for pharmaceutical companies, enabling them to address challenges […]

Robotics in Healthcare: Revolutionizing Patient Care
Authors: Robin Arnold, Ankit Kulshrestha, Abheek Bose, Mayur Muley, Anant Kasodekar The launch of the PUMA 650 surgical robot in the 1980s, initially employed in brain biopsy procedures, ushered in the era of medical robots.1 From then until the present day, remarkable innovations have led to the development of state-of-the-art robots and their seamless integration […]

Navigating the Evolving Landscape of Internet Healthcare in China: Development Status, Challenges, and Future Prospects
Authors: Robin Arnold, Executive Vice President; Renyang Liu, Engagement Manager; Erica Wang, Associate Consultant Background Internet healthcare means using the internet to provide health and medical services. China has implemented “Internet Plus Healthcare” as a national policy, as a part of reforming the healthcare system. Internet healthcare in China is estimated at 150 billion RMB […]

Making Primary Market Research SMARTER Through Social Listening
Discover how EVERSANA is transforming the landscape of primary market research in the pharmaceutical industry through our unique approach to social listening. This insightful article from our APAC team highlights the common challenges faced by pharma companies and how most fall short in translating social insights into actionable strategies. EVERSANA, on the other hand, stands […]

APACMed Digital Health Reimbursement Policy Forum
Introduction The APACMed Policy Forum on Digital Health Reimbursement was held on 23 May 2023, with participants from Australia, Japan, Singapore, South Korea, Germany, France, and the UK. Several digital health policymakers, academicians, and experts from the respective countries, along with the APACMed Digital Health Reimbursement Alliance (DHRA) core committee, local trade associations, and EVERSANA […]

The Rising Demand for Light Medical Aesthetic in China
“The Rising Demand for Light Medical Aesthetic in China” provides a captivating insight into the surging popularity of non-surgical medical aesthetics in China. The article highlights the rapid growth of the medical aesthetic market and the shifting trends in consumer preferences. It delves into the key contributors and experts driving this industry and examines the […]

Revolutionizing Vaccines: The Rise of mRNA Technology in China
Key Contributors: Robin Arnold, Executive Vice President; Renyang Liu, Engagement Manager; Nicole Li, Analyst Discover how mRNA technology is transforming the field of vaccines in China. From the evolution of vaccine development to the rapid progress of mRNA vaccines during the COVID-19 pandemic, this article explores the advantages of mRNA vaccines compared to conventional ones. […]

Commercial Key Success Factors (KSFs) for Global Drug Development Programs
Key Contributors: Swapnil Waichale, Principal and Robin Arnold, Executive Vice President Discover the essential commercial key success Factors (KSFs) that drive triumph in global drug development programs. In an era of diverse regulatory perspectives and reimbursement complexities, biotech companies face higher stakes than ever before. Swapnil Waichale and Robin Arnold unveil the top failure modes […]

The Role of RWE in Expediting the Drug Approval Process
Key Contributors: Lydia Edison (Project Manager), Rohit Mandlik (Project Manager), Rohit Dang (Engagement Manager), Mahendra Rai (Senior Director) The role of real-world evidence (RWE) in expediting the drug approval process. RWE refers to the use of real-world data (RWD) from sources outside traditional clinical trials to support regulatory decision-making. RWE offers several benefits, including generating […]

Position Your Future-Facing Strategy on Digital Therapeutics (DTx) in China
How can DTx resonate with current portfolios and capture incoming opportunities? Key Contributors: Robin Arnold, Executive Vice President; Jerry Song, Associate Principal; Qiwei (Alex) Li, Consultant The article discusses the potential of digital therapeutics (DTx) in China’s healthcare system and how it can help pharmaceutical and medical technology companies address the challenges presented by the country’s aging […]

A Comparison of Existing Real World Evidence (RWE) Guidelines
Key Contributors: Neel Patel, Associate Consultant- HEOR, APAC and Rajanikanth Manupati, Associate Consultant- HEOR, APAC In the fast-paced world of healthcare, where the development and approval of new therapies can take over a decade, the need for modern solutions is more pressing than ever. Enter Real World Evidence (RWE) – a game-changing approach that harnesses […]

Navigating the Complexities: Strategies for Asian Pharma Companies Entering the U.S. Market
Key Contributors: Divayum Gupta (Analyst), Tanay Sharma (Consultant), Sowbhagya Suresh (Senior Consultant), Jinsol Kim (Engagement Manager) The United States holds a dominant position in the global pharmaceutical market, accounting for 45% of global sales in 2022. However, Asian pharmaceutical companies face significant challenges when entering the complex U.S. market. The U.S. healthcare system involves multiple […]

Pricing Complexities of a Combination Therapy
This article, authored by EVERSANA’s Asia Pacific team, discusses the increasing use of combination therapies in oncology and the challenges associated with pricing them. Combination therapies, which involve a backbone therapy and one or more add-on therapies, offer better clinical outcomes but pose difficulties in determining their value and pricing. One challenge arises from the fact […]

2023 Outlook Of China Healthcare Industry: Remaining Pragmatic While Moving Up The Value Chain
As we enter 2023, China surprised the world with a swift and decisive shift in COVID policy, demonstrating the country’s ability to be flexible and pragmatic in its decision-making. In contrast to Russia’s persistent adherence to ideologies, China demonstrated its willingness to pivot quickly when necessary. The 14th Five-Year Plan at the 20th National Congress […]

Adult Vaccine Landscape in APAC: The Need for Change
Content for this article was contributed by EVERSANA’s Asia Pacific team. Do Adults Need Vaccines? Immunization should not end with adulthood. Using immunization to prevent infection should be a life-long process, as adult vaccination provides benefits at both individual and country levels, reducing the overall burden of preventable diseases. However, in practice, adult vaccinations are […]

Access Challenges for Severe Infectious Diseases
Content for this article was provided by EVERSANA’s Asia Pacific team. The global burden of infectious diseases is increasing, especially for severe diseases such as invasive aspergillosis, a rare infectious disease, which has a 90% mortality rate. These diseases have poor prognoses and high mortality rates. Disease severity and mortality rates have been increased by […]

Evolution of Digital Apps to Total Wellness Solutions
Content for this article was contributed by EVERSANA’s Asia Pacific consulting practice. There is a remorseless demographic logic to increasing health costs. Life expectancy increases, the population ages, and “lifestyle” diseases penetrate further and earlier. How can public health stakeholders resist the trend? One intervention point is promoting the benefits of healthy, active lifestyles. Government-backed […]

The Evolution of HTA and Its Impact on Drug Prices in Japan
Health Technology Assessment Status in Japan Health technology assessments (HTAs) are increasingly used worldwide to assess the clinical and economic impact of drug treatments and technologies, inform health policy, and guide drug pricing and reimbursement. In Japan, HTA occurs after the debut of a product and when companies enter the system, whereas in the UK […]

Plasma derived medicinal products – What is the way forward?
Content for this article was contributed by the EVERSANA Asia Pacific team. Plasma-derived products have a long history of providing benefits, especially in fractions addressing coagulation. Plasma is a rich biological substance and one of the main components of blood, constituting about 55% of total blood volume. The worldwide plasma protein market is expected to […]

Single-Cell Multiomics: Opportunities, Challenges, and the Evolving Business Model
Content for this article was contributed by EVERSANA’s Asia Pacific team. Single Cell Multiomics is a Fast-Growing Market Space with a Wide Range of Applications Single cell multiomics is a new and exciting space in the life sciences industry and research community. Advances in single-cell isolation and barcoding technologies have provided opportunities to profile DNA, […]

Successful Early-Stage Out-Licensing Requirements
The attention on vaccines during the COVID-19 crisis highlighted the role of biotech companies and positive sentiment increased for the sector. In 2021, a record number of biotech (100+) companies went public and listed companies soared to an all-time high. However, today investors have reset their expectations for the sector considering the current economic trends. […]

Is Telemedicine Here to Stay?
Prior to the COVID-19 pandemic, healthcare delivery had been predominantly face-to-face. COVID-19 exposed the capacity limits of the healthcare system, and forced the system to optimize its methods of healthcare delivery by considering non-conventional methods such as telemedicine. Download our article to learn how our telemedicine matrix can inform companies’ mid-to-long term brand strategies.

Established Pharma Brands – A Good Investment?
Offering high cash flows with low clinical and commercial risks, established brands are gaining attention. Big pharma, such as Merck, GlaxoSmithKline (GSK), Pfizer and Sanofi, are spinning off their established brand drugs into new companies. Merck announced that it would be creating a new company, to house its women’s health, well-known established brands and biosimilar […]

An Innovative Approach to Comprehensive Key Opinion Leader (KOL) Mapping
Traditional key opinion leader (KOL) mapping techniques are heuristic and often haphazard: EVERSANA™ has developed an innovative and robust approach that dramatically improves results. We base our ability to identify and prioritize KOLs across disease areas on new technologies and datasets that have made it possible for us to customize searches for many client objectives, […]

Evolution of Integrated Mental Health Treatment Models in Southeast Asia (SEA)
Implement Mental Health Integrated Care Models with A Trusted Partner Integrated-care models for mental health conditions implemented in China and Japan have demonstrated the efficacy and cost-effectiveness of such models. SEA is the next frontier of implementing these models and stakeholders in SEA countries have begun assessing and implementing such models. However, infrastructure, numbers of […]

Challenges of Scaling Up Cell Therapy to New Geographies
Cell therapy has emerged as one of the most promising disruptive innovations in the pharmaceutical industry. However, despite its commercial promise, companies developing cell therapies have not been able to take full advantage of its massive potential. Cell therapies offer a massive commercial opportunity In 2021, the cell and gene therapy (CGT) market was valued […]

Booming but Constrained: Digital Therapeutics for Mental Health in APAC
Digital therapeutics (DTx) are a fast-emerging class of therapies that use software to treat disease, both as standalone treatments and for treatment optimization. Applications range from improving patient adherence to supporting physicians in managing patients remotely. DTx has been much hyped as transformational across the healthcare ecosystem by bringing personalized medicine to all through AI […]

Need for RWE in Consumer Health and Its Impact in Managing Lifecycle of Consumer Health Products
Healthcare has never been more important to the world than in the past two years and it will remain central during the economic recovery. The pandemic’s impact on medical and healthcare systems has emphasized the value of real-world evidence (RWE). This value is now becoming apparent in consumer health, as well as its traditional applications […]

Reframing the Challenges of Access and ROI
Pharma companies today are being judged not just on their profitability for shareholders, but also on the extent to which their products are accessible to patients. Today’s Environmental, Social and Governance (ESG) investors want to see evidence of corporate responsibility, for which pharmaceuticals’ surrogate measure is access. While companies such as Novo Nordisk and UCB […]

The Need for RWE Studies in APAC and the Impact on Product Life Cycle Management
Advances in technology and globalization have enabled significant growth in the pharmaceutical industry over the last decade. Digital initiatives and focusing on patients have also conditioned researchers to think beyond traditional data collection to support product value propositions. As a result, real-world evidence (RWE) is gaining in importance and becoming an important part of managing […]

Health Technology Assessment in the Asia Pacific Region
Health technology assessment (HTA) is the systematic evaluation of the properties and effects of a health technology, addressing the direct and intended effects of the technology as well as its indirect and unintended consequences. In many countries, it is a primary evaluation tool for making pricing and access decisions, providing a platform for understanding economic […]

Building a Life Sciences Digital Ecosystem
In the life sciences world, we hear more and more of the requirement for digital ecosystems in various therapies. What do we mean by these, and what benefits do they provide? A digital ecosystem is a network of multiple stakeholders who collaborate to enhance delivery to patients. A healthy, sustainable ecosystem benefits patients, prepares companies […]

Assessing Market Opportunity and Entry Challenges in Mental Health Disorders
Although mental health disorders affect almost 264 million people worldwide, they are often misunderstood and misdiagnosed, and access is poor. The World Health Organization (WHO) reports that 80% of people in low- and middle-income countries receive no treatment for their mental health disorders. As well as being underdiagnosed, mental health disorders are also poorly studied […]

Increasing the Value of Community Health Workers
In developing countries, frontline community health workers (CHWs) extend basic healthcare services to communities. These extensive CHW networks provide services that improve various health indicators. The Philippines has set a goal of one CHW (“Barangay Health Worker”) in every village, while India is close to meeting its goal of one CHW (“ASHA”) per 1,000 people […]

Novel Antibiotic Opportunity in China
China, with its large population and extensive healthcare system, is the second-highest user of antibiotics in the world. Such widespread use has inevitably led to high levels of antibiotic resistance in the population. When isolated, it becomes clear that a significant majority of the infecting bacteria are gram-negative. Within these bacteria, antibiotic resistance has grown […]

Digital Therapeutics: Where Technology Meets Healthcare
Digital health is growing rapidly across the world, supported by technologies that not only assist disease monitoring or prevention but can also reduce the healthcare burden. Digital technologies have improved equity of access and enabled European patients to monitor and self-manage their health. It has improved efficiency, effectiveness and quality of patient care while also […]

Adapting Patient Access Programs to Asia
In Western markets, pharmaceutical companies have developed many approaches to patient access programs (PAPs), normally emphasizing initiatives that, while helping patients, also support pricing and reimbursement initiatives, such as: Early access programs before market authorization. Financial access initiatives before achieving reimbursement. Value-based contracts, managed-entry agreements or outcomes-based programs for achieving reimbursement. Non-financial assistance post-reimbursement. The […]
The 10-Year Test: Is It Possible to Plan Launch During Clinical Development?
Time and strategy are keys to launching in an overwhelmed, unpredictable market. Pharmaceutical companies in mid-development of a drug need to look ahead at their launch strategy options and consider what market conditions could look like by the time they’re ready to commercialize their product. By evaluating the value of an asset early in development, pharma companies can gain a better understanding of a product’s value and risk. With assessment insights, manufacturers can make informed clinical and commercial […]

Case Study: Early-Stage Asset Valuation
The rewards of developing a new drug can be very attractive but they come with inherent risks. It is important to ensure that investment decisions on clinical development are made after examining the risk profile of the drug which depends on the therapy area and the nature as well as extent of unmet needs. EVERSANA […]

The Importance of Digital Strategy in Pharma in the APAC Region
The Asia Pacific region continues to show great progress in digital health as pharmaceutical companies look at digital technologies as a tool to drive access and to start developing their digital strategies. To understand the strides and recent evolution of digital in pharma, our APAC team conducted a survey with top pharmaceutical companies in the […]

Improving Patient Outcomes Through Digital Health
Digital applications can work cohesively with therapeutics in the treatment of a broad range of diseases, from behavioral health to chronic conditions. In an interview with Debraj Dasgupta, Head of Strategy & Go-to-Market Planning at Nippon Boehringer Ingelheim Co., Ltd., Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, […]

The Digitalization of Healthcare in Asia Pacific
The digitalization of healthcare in Asia Pacific is being utilized to enhance the patient-centered approach and expanding access to health. In a candid conversation with Abhishek Shah, CEO at Wellthy Therapeutics, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, uncovered the current healthcare landscape and how digital strategy […]

The Regulatory Landscape in Digital Health
Despite the advances in digital health and digital therapeutics in recent years, there remains an ambiguity in the digital space and uncertainty surrounding regulation and reimbursement of digital technologies applied to pharma, biopharma and medical device companies. In an insightful conversation that was part of EVERSANA’s Digital Symposium, Ed Cox, Executive Vice President, Strategic Alliances […]

Access to Technology and the Rise of Digital Health in Asia Pacific
The access to technology, development of telehealth and evolution of digital therapeutics are thriving in Asia Pacific; and pharmaceutical companies are levering the momentum to reach to a broader segment of the patient population. Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, interviewed Anish Shindore, Head of Digital […]

Digital Innovation in Asia Pacific
Digital medicine and digital therapeutics are transforming the healthcare industry by changing the care delivery format, and they have the potential to improve patient adherence and outcomes. In our recent Digital Symposium, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine at EVERSANA, interviewed David Keene, CEO at IntroSpect Digital Therapeutics […]

Outlook: Digital & Pharma in Asia Pacific
Asia Pacific is one of the fastest-growing regions for digital innovation in the pharmaceutical and biotechnology industries. Its promising outlook is mainly driven by necessity, commercial flexibility and a broad talent pool. In a candid conversation broadcasted during the EVERSANA’s Digital Symposium, Ed Cox, Executive Vice President, Strategic Alliances & Global Head of Digital Medicine, […]

Leading and Decision-Making in Uncertain Times
By definition, leaders must have followers, who are defined as people who must be shown the way. While this is within most leaders’ grasps when the road ahead is straight and wide and the destination is visible, more is demanded of them when the road splits, narrows and dusk falls. The current crisis presents special […]
Realizing Value from Digital in the Healthcare Environment [Webinar]
[WEBINAR – 20 MINUTES] The advancements of Digital Therapies are promising but commercial success is not always guaranteed. This presentation explores the pitfalls and challenges of selling products into the uniquely complicated and regulated healthcare market. It will lay the groundwork for digital companies to have successful commercial strategies and be able to: Answer questions […]

The New Roaring 20s: How Outsourced Commercialization is the Revolution We Need
The “Great Gatsby Era” was an exciting time of new prosperity, infinite creativity and dramatic social change. Long-standing social norms and traditions gave way to the “mass culture” and consumerism that modernized American society. “What will they think of next?” was a popular expression that defined the era. On the 100-year anniversary of this revolutionary […]

Improving the Impact of an Oncology Patient Access Program
Our client, a specialty pharmaceutical company focused on the research and development of new therapies in two high-cost complex drug categories, sought to benchmark its patient assistance program against those of its competitors in order to improve relationships within target oncologists’ offices. The Challenge The client needed to assess the image of its patient assistance […]

Strategies to Ensure Market Success of a Rare Disease Product Launch
Small patient populations, complex administration, high costs of therapies, and government policy interventions are just a few obstacles in rare disease. EVERSANA’s Managing Director, Bill O’Bryon, and Senior Director, Diann Johnson, outline key insights to consider in your launch strategy: Market dynamics impacting rare disease therapies The entire rare community: patients, caregivers, providers & advocates […]

After FDA Approval, Can Gene Therapies Achieve Marketing Success?
Without a doubt, gene therapy is transforming healthcare by revolutionizing patient care from conventional treatment models to curative therapy models. There are more than 7,000 distinct types of rare and genetic diseases and 400+ million individuals suffering from a rare disease. With a market that is fragmented, there is a need for an innovative, end-to-end […]

Global Biosimilar Competitor Assessment – Who Wins in Biosimilar Access and Why?
The introduction of biosimilar products has created interesting changes in payer behavior across the global markets. For both innovator and biosimilar companies, it has been an effort to understand the demands of payers and succeed in what looked like a ‘winner-takes-all’ opportunity. As the first round of biosimilar introductions has passed, there are some interesting […]

Innovative Therapies Call for an Integrated Drug Safety and Compliance Model
In this article, Herbert Lee, PharmD, Senior Vice President, Medical Communications and Pharmacovigilance, makes the case for an integrated approach to ensuring the safe and effective use of medications by patients. From emerging therapies to innovative technologies, the healthcare industry is changing – demanding that we do business differently. Working in one of the most […]

Gene Therapy for Rare Disorders
With only three approved products in the marketplace, how do we build a regenerative medicine ecosystem that delivers more value to patient’s faster?
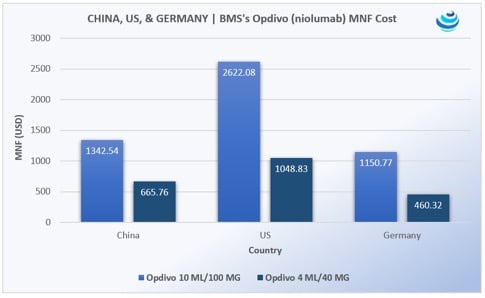
‘Tis the season for pharma in China, as the government expedites uptake of new urgently needed drugs
PRICENTRIC BRIEF: Universal health insurance coverage for 1.3 billion people means China must increase uptake of “clinically urgently needed new drugs” already approved in the US, EU, and Japan Mostly oncology products are being imported, along with HIV/AIDS medicines Pharma is agreeing to steep discounts to be reimbursed on China’s national drug lists THE DETAILS […]

