27 Markets, 1 Unified Launch Strategy
Launching in Europe can be complex. With 27 countries and profound differences between them, how can you ensure an optimal launch?
Traditional approaches to launch sequencing need re-evaluation due to multiple changes occurring across the region. Countries are increasingly interconnected, with decisions taken in one market having a ripple effect across multiple markets.
Reduce Risk with a Comprehensive European Launch Strategy
Price, reimbursement, and access are critical to commercial readiness in European markets.
Without a strategy to address all three, pharma and biotech companies have limited success in what is a critical market. That’s why EVERSANA has created a fixed-fee“European Commercial Readiness Opportunity Assessment” to help companies develop optimal launch plans, powered by deep market expertise and propriety data & software powered by our industry-leading NAVLIN by EVERSANA platform.
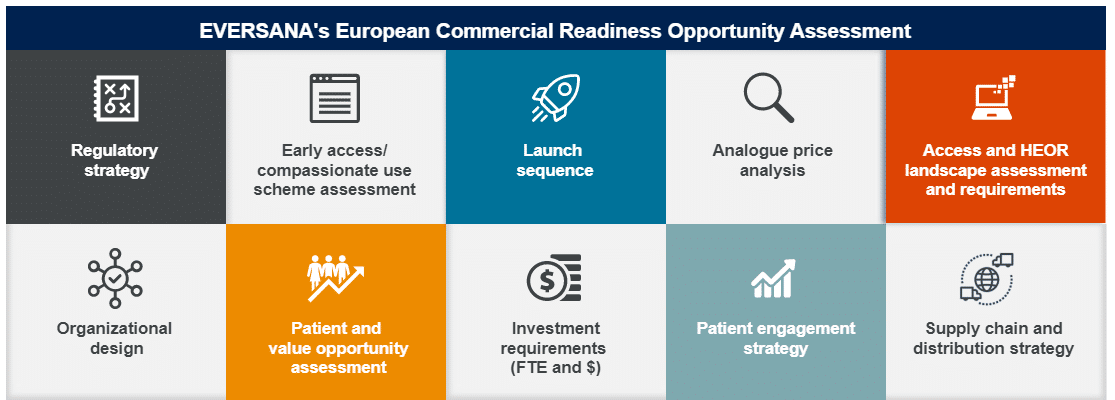
Powered by data and software, we can help you deliver stakeholder value with:
- Regulatory strategy
- Analogue price analysis
- Access & HEOR landscape assessments
- Supply chain & distribution strategy
- And more
EVERSANA’s team of EU launch experts can help you optimize your launch with early planning, market understanding, entry strategy, and launch sequence optimization.
Contact us today to learn more about how we can support your commercialization in Europe.




