On March 5, 2024, the draft Implementation Act (IA) for Joint Clinical Assessment (JCA) for medicinal products was released for public comments. The intent of the draft IA was to provide more granular details on the JCA process within the framework of the Health Technology Assessment Regulation (HTAR).
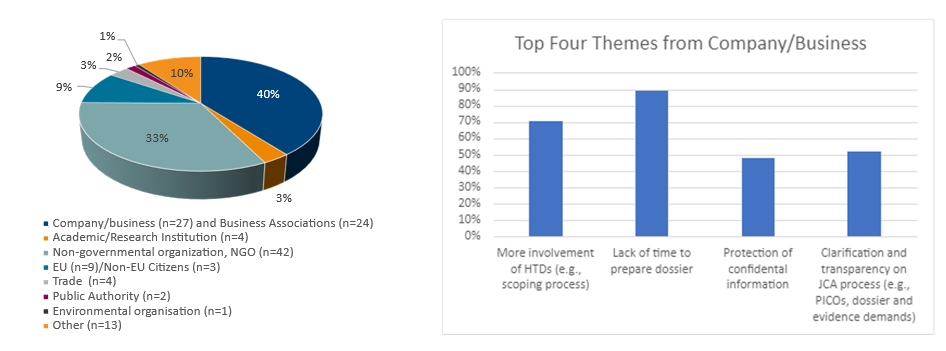
By the April 2, 2024, deadline, 129 responses by various stakeholders were received. The responses were categorized by organizational types, which included: Company/Business, Business Associations, Academic/Research Institutions, Non-governmental Organization (NGO), EU/Non-EU Citizens, Trade, Public Authority, Environmental Organization and Other.
The Company/Business (21%) and Business Associations (19%) together comprised 40% of the responses. Among the respondents from the Company/Business stakeholder, the No. 1 issue was the lack of sufficient time to prepare a high-quality dossier. This was followed by including health technology developers (HTDs) throughout the JCA process, particularly during the scope phase. The third- and fourth-most-mentioned themes centered on the whole JCA process and confidentiality, respectively.
Following the public consultation period, an updated draft JCA IA document was released on April 26 incorporating the top themes. In the latest version, the dossier submission timeline was increased from 90 days to 100 days. HTDs now can request a scope explanation meeting rather than waiting for an invitation from the HTA secretariat (if the JCA subgroup deemed it necessary). Finalization of the scoping assessment was decreased from 100 to 75 days following the EMA validation of the Market Authorization Application. In addition, HTDs will have the opportunity to redact confidential information.
The EU HTAR intent is to minimize duplication efforts and harmonize assessment across the EU members states, with the ultimate goal of improving patient access by providing faster and more equitable access to medicines across EU. However, before reaching this goal, there will be challenges to overcome to optimize the EU HTAR and JCA process. Although the updated JCA IA draft incorporates feedback from the public consultation, there remains an increased demand for evidence, strain on company resources and uncertainty regarding the practical implementation of HTAR (despite revisions in the timelines).
EVERSANA can help you overcome these challenges by developing a robust evidence package for a successful dossier submission. Our comprehensive support includes, but is not limited to:
- Development of HTA dossier
- Burden of disease (e.g., SLR/TLR, epidemiology)
- Comparative data analysis (e.g., ITCs) aligned with anticipated PICOs
- Early stakeholder engagement (e.g., patients, physicians, payers)
At the EU member states level, we also provide:
- Economic models (e.g., CEA, BIM), including economically justifiable price
- Cost-of-illness studies
- Local dossiers (including economic evidence)
- RWE generation
For more details on the concerns highlighted by IA JCA comments, consider partnering with EVERSANA. We can help you prepare for and overcome these challenges – particularly the ambitious dossier timeline!
Author

Pamela Vo brings over 18 years of HEOR experience in the pharmaceutical industry, showcasing a history of strategically translating ideas into impactful outcomes. She boasts a proven track record of peer-reviewed publications, significant achievements…

Tim is an experienced analyst and emerging thought leader in the application of Bayesian methods to complex problems. His Vanier Canada Graduate Funded dissertation research focused on the incorporation of multivariate evidence synthesis in…

