Considerable effort is dedicated to inputting clean adverse event data into a safety database, even before conducting the analysis to extract insights. In this scenario, manufacturers are investing more resources in the process of inputting data from various sources than analyzing and researching the information. To right-size where the primary focus is, manufacturers must adopt a high-tech high-touch approach to pharmacovigilance that blends automation and human interaction.
Fear frequently stands as the primary barrier linked with change, arising from an inadequate comprehension of a process and a reluctance towards the unknown and its associated risks. However, if there is an opportunity to enhance efficiency, improve quality and adapt to evolving needs, refusing to embrace change can result in a loss that accumulates over time.
Think about it this way: the resource efficiencies gained from embracing change can be redirected towards more meaningful activities that can significantly increase profits. Technology enables the automation of repetitive and time-consuming tasks in pharmacovigilance, allowing human resources to be allocated to more strategic and value-added endeavors. To put it simply, here’s a profit-loss balance example for this concept:
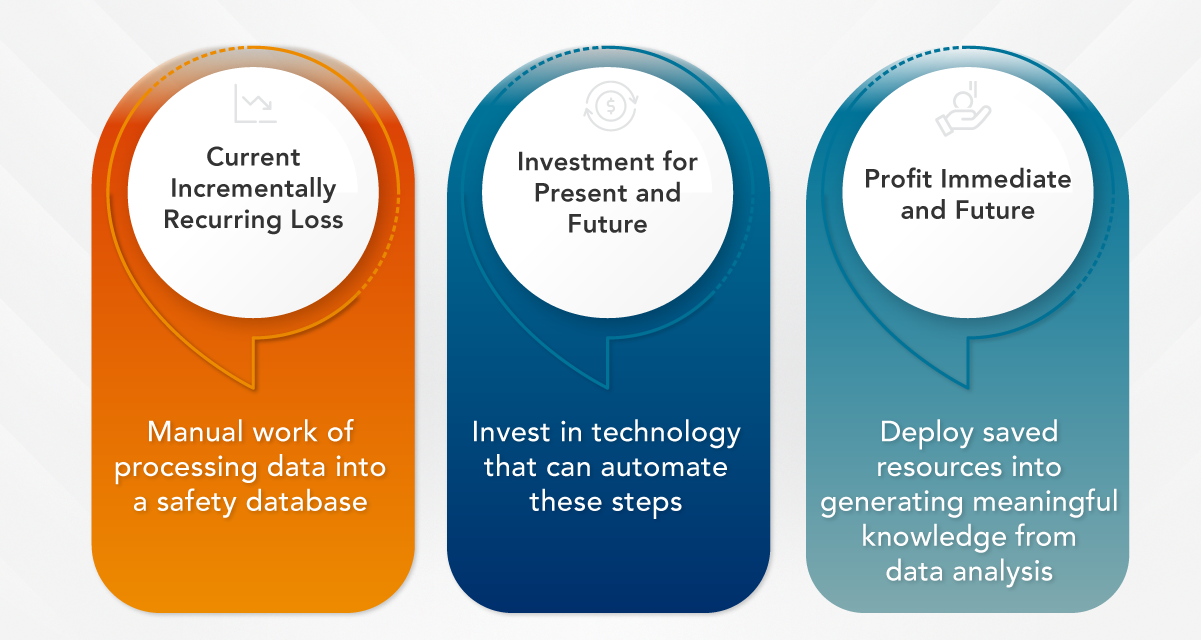
EVERSANA’s Integrated Pharmacovigilance Solution fuses cutting-edge technology with human interaction and minimizes risk for manufacturers with its flexibility and proven capabilities. Activities formerly managed by humans have been automated, liberating resources for crucial elements such as translation review, quality assurance and medical assessment. The market-disrupting element this solution offers is that the customer need not access the automation tool at all because EVERSANA – through this Integrated Solution – will deliver the processed AE data fully compliant and quality assured per regulatory requirements.
The solution stands as a beacon of adaptability, tailored to suit the unique demands of various organizations and proprietary databases, stages within the product life cycle and disease states. Backed by a track record of ongoing successful implementation with five diverse clients of EVERSANA, its capabilities have been proven through meticulous validation testing and comprehensive hazard analysis. Machine Learning benchmarks, including Precision, Recall, and F1 Scores attest to its reliability.
Author

Dr. Vivek Ahuja serves as EVERSANA’s Senior Vice President for Delivery Excellence, Strategy, and Growth (PV, Quality, and Regulatory Services) with over 22 years of experience across multiple functional offerings including Pharmacovigilance, Clinical Research,…

