
Posted on 10月 3, 202310/3/23
FDA Inspection Win For a Mid-Sized Pharma Company with More Than 40+ Products in the USA and Outside the USA

Posted on 8月 24, 20238/24/23
Representative Engagements: Patient Access Platform for Digital Medicine Device
Posted on 7月 3, 20237/3/23
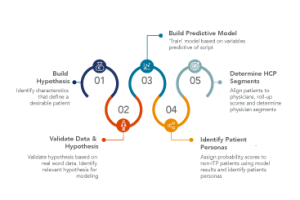
Identifying Rare Disease HCPs with Omnichannel Targeting
Posted on 6月 23, 20236/23/23
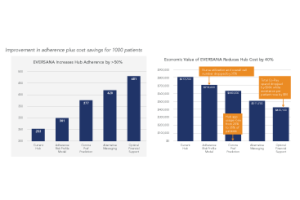
Improving Adherence & Compliance Via Patient Targeting

Posted on 6月 23, 20236/23/23
Oncology Launch Scenario Planning

Posted on 6月 23, 20236/23/23
Defining the Rare Disease Patient Journey to Support Commercialization of Therapy

Posted on 5月 1, 20235/1/23
Optimizing Launch & Maximizing Revenue Potential in Europe

Posted on 11月 10, 202211/10/22
High-Tech Medical Information Services

Posted on 11月 1, 202211/1/22
Client + Vendor Partnerships Achieve Strategic Objectives

Posted on 11月 1, 202211/1/22

