EVERSANA is a proud to sponsor the World Orphan Drug Congress USA held at the Boston Convention and Exhibition Center April 23-25. Visit our booth or schedule a meeting with EVERSANA’s experts in rare diseases, patient services, data and analytics, and commercialization services to learn how EVERSANA leads the way in simplifying complex rare disease patient journeys.
Don’t miss the panel discussion and presentations below featuring experts from EVERSANA on Wednesday, April 24:
Unlocking Patient-Centric Insights and Feedback to Elevate Engagement
- Panelists:
Maria Kirsch, President, Patient Services, EVERSANA
Patricia Weltin, CEO, Beyond the Diagnosis
Joshua Resnikoff, CEO, TMA Precision Health - Moderator: Anthony Scatamacchia, SVP, Patient Services, EVERSANA
- Time: 1:35-2:15 PM
- Location: Great Hall C
- Description: Join us for a dynamic panel where experts from diverse backgrounds delve into real-world examples improving the lives of rare disease patients. From Maria Kirsch’s insights on the impact of white-glove patient support to Patty Weltin’s fierce advocacy, and Josh Resnikoff’s mission to diagnose patients faster, our discussion promises inspiring perspectives.
The Use of Machine Learning to Improve Time to Diagnosis of Patients with Probable MPS II: A Case Study
- Presenters: Dr. Pierantonio Russo and Ramaa Nathan, PHD
- Time: 3-3:20 PM
- Description: EVERSANA’s Dr. Pierantonio Russo, Chief Medical Officer, and Ramaa Nathan, VP of Data Science and Real-World Evidence, will share how data-driven insights can help identify the unmet needs of patients with rare diseases and their healthcare providers, thereby facilitating the development of a compelling value proposition for therapy. Through this case study, hear first-hand how historical data from open claims, map referrals to key events along the treatment pathway, and graphing networks connecting providers by volume of patients were used to identify the top referring providers and high value treatment centers and catchment areas responsible for over 50% of patients with a particular rare disease in their respective regions.
Health Technology Assessment of Rare Disease Drugs: Exploring Willingness to Pay
- Presenter: Lindy Forte
- Time: 11:45 AM-12:05 PM
EVERSANA’s Rare Disease Expertise
With over 7,000 distinct rare and genetic diseases affecting 400+ million individuals, the average time to diagnosis still remains approximately 7 years. In response, EVERSANA’s end-to-end commercialization platform provides manufacturers innovative solutions to accelerate speed to market, diagnose patients faster, simplify new therapy onboarding and personalize adherence support.
By leveraging data and AI capabilities of ACTICS by EVERSANA® across the patient journey, EVERSANA can predict patient switching behaviors that impact adherence and engagement. This information also powers the optimization of our hub and affordability programs and deploys the “next best action” for each patient, leading to up to a 50% patient adherence improvement over a traditional hub program.
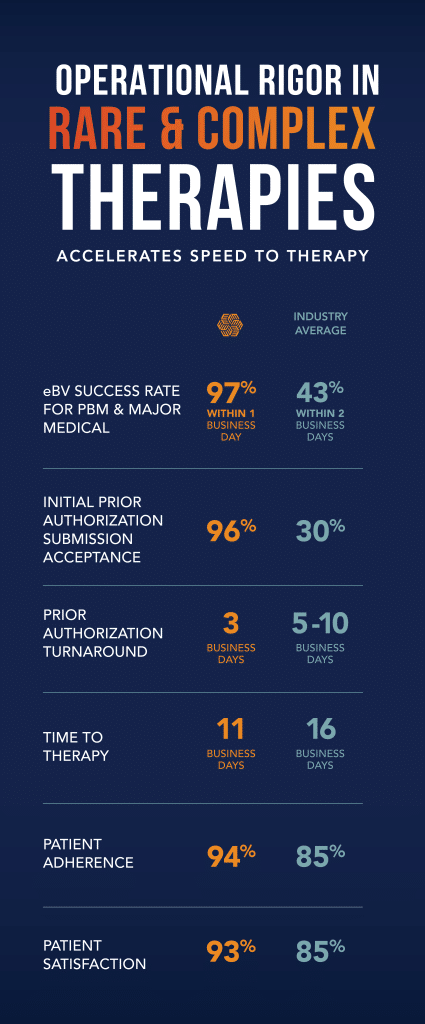
EVERSANA’s Patient Services simplifies and expedites the patient journey. Our hub accelerates patient onboarding by supplying real time eligibility determination, self-enrollment with support options and e-prescribing. Additionally, ACTICS® eAccess, EVERSANA’s new proprietary eBV and ePA platform, verifies patient coverage for Pharmacy Benefit Manager and Major Medical with a +90% success rate in seconds rather than days.
Our specialty pharmacy model connects patients, providers and payers to offer direct-to-patient dispensing and administration. In addition, data-driven patient profiling can determine customized outreach to provide education and deliver white-glove support to patients, driving brand loyalty and adherence.
Read Our Latest Thinking on Rare Disease Commercialization
- Digital Guide to Commercializing Complex Therapeutics
- Navigating Routes to Commercial Excellence in Cell and Gene Therapy
- Critical Success Factors for Launching Products with Orphan Drug Designation
- Case Study: Leveraging machine learning models in patient identification and behavior prediction for rare disease indications
- Case Study: Defining the Rare Disease Patient Journey to Support Commercialization of Therapy
Learn More About EVERSANA
PATIENT-CENTERED SERVICES
Surrounded by best-in-class field deployment, agency, channel management and more. Explore more:
- Innovation for Rare and Orphan Drugs
- Patient Services
- Specialty Pharmacy
- Electronic Benefits Verification (eBV) and Electronic Prior Authorization (ePA)
- ACTICS® by EVERSANA
- Data and Analytics
REINVENT COMMERCIALIZATION
EVERSANA has reinvented commercialization in the pharmaceutical space. We work with large pharma to optimize the last few years of established brands – making the most out of revenue potential. We are working with emerging biotech to launch their first products – delivering unparalleled agility to navigate market complexities and drive down costs – up to 20% if they had to commercialize on their own.
- Operational Rigor
- Integrated Services
- Optimized by Analytics
NEW MODELS FOR ONCOLOGY, RARE & HIGH SCIENCE BRANDS
We understand the needs and sophistication of complex therapies. Our award-winning commercialization models were developed to be agile, flexible, cost effective and to lower the risk of uncertainty. They are digital first and powered by data, analytics and AI.
- Agility to pivot strategies without “change order” mentality
- Speed to market
- Risk management
- Owners maintain full value of their asset
Visit our booth schedule a meeting with EVERSANA’s rare disease experts today.
About World Orphan Drug Congress
The World Orphan Drug Congress brings together leading pharmaceutical and biotech companies, government and regulatory authorities, patient advocacy groups, payers, investors and solution providers. The conference is a place to meet and brainstorm ways to advance orphan drug development and improve access to life-saving therapies.

