Posted on December 6, 202312/6/23
Linking Channel Distribution & 3PL to Non-Traditional Commercialization Touchpoints

Posted on September 7, 20239/7/23
Balancing the Portfolio: How Extending Mature Brands’ Success Can Replenish Costs Invested In Launching New Products

Posted on September 7, 20239/7/23
Asset Optimization: Breathe New Life Into Mature Brands with EVERSANA REIGNITE™

Posted on August 21, 20238/21/23
Maximizing Product Launch Success: The Importance of Conducting Comprehensive Business Simulation Exercises

Posted on June 28, 20236/28/23
Forecasting in the Age of Value-Based Agreements

Posted on June 28, 20236/28/23
Innovative Approaches to 340B Claims Data Identification

Posted on June 20, 20236/20/23
Key Considerations When Operationalizing Revenue Management

Posted on June 16, 20236/16/23
Digital Guide to Commercializing Complex Therapeutics
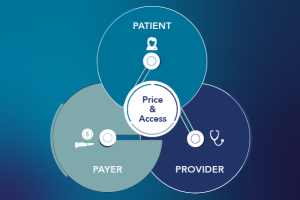
Posted on June 12, 20236/12/23
Informing Optimal Pricing Strategies with a Comprehensive Modeling Approach

Posted on February 23, 20232/23/23

