Insights
Insights From Our Experts
Articles

Italy Tackles HTA, Pricing and Reimbursement Reforms
In July, new legislation set to overhaul the way pharmaceuticals are priced in Italy was finally implemented, following an announcement that dates all the way back to August 2019. The provision, which was signed by then…
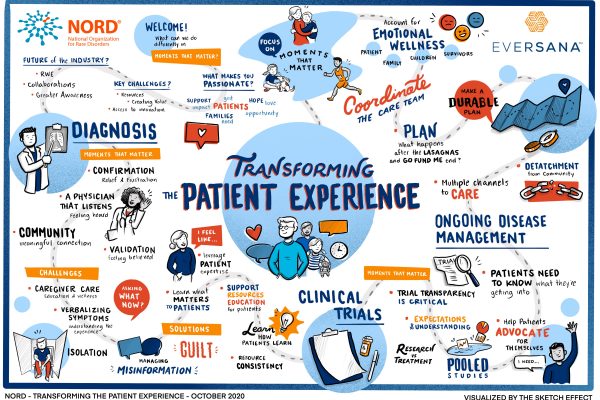
An Illustration: Transforming the Patient Experience
As a fierce ally of the rare disease community, EVERSANA proudly sponsored the 2020 Rare Diseases & Orphan Products Breakthrough Summit. Longtime partners in the advancement of orphan drug commercialization, EVERSANA and the National Organization for…

eBook – 2020 Stories That Shaped Commercialization
While many industries hit the pause button on their operations this year, the pharmaceutical industry never stopped, propelling new innovations to better serve patients, providers and stakeholders. At EVERSANA, we worked closely with manufacturers to tackle…

When A Curveball Threatens Your Product’s Launch
Download the article to learn about proven strategies to help shield your company from unwelcome surprises during launch by reducing your financial exposure and creating a more stable and flexible launch operation.

CBI Hub, SPP and eServices Insights: Leveraging Behavioral Health Technology
The Science of Human Resiliency — Behavioral Health Technology to Improve Patient Outcomes and Adherence Over the past several years, pharmaceutical manufacturers have been in pursuit of improving the patient experience by reducing time-to-treatment, developing a…

PharmaVOICE Commercial Influencers: Greg Skalicky
In a special focus on Commercial Influencers, PharmaVOICE Editor Taren Grom sat down with EVERSANA’s Chief Revenue Officer Greg Skalicky to discuss approaches to commercial strategies and how disruptive companies are changing the market to better reach and engage patients. The conversation covered commercialization trends, how…

Advancing Beyond the Traditional Patient Adherence Model
Showcase Featured In PharmaVOICE – October 2020 Delivering value in the era of empowered patients renders the one-size-fits all patient services program obsolete. Not every treatment journey is consistently linear within a disease state because patients…

Compliance – The Competitive Differentiator to Commercialization: An Integrated Model Setting the New Global Standard
The Compliance experts at EVERSANA prove how integrated life science compliance has earned its seat at the table by demonstrating the value of increasing data-driven and technology-infused competitiveness in the successful commercialization of a new-age biopharma…
Trends in HTA Decisions
Alan Crowther and Dr. Magdi Stino explore trends in HTA decisions from the Pricentric HTA database for all therapeutic areas and within oncology. Examining over 25 HTA bodies from 2011 to 2019, they present findings on how…

WODC Insights: Developing HEOR Programs for Orphan Drugs
To successful launch a new orphan drug, demonstration of therapeutic success is crucial for a global HTA submission and securing market access. Manufacturers need to create a compelling value story with real-world data to illustrate how…

