Key Benefits of the EVERSANA ONCOLOGY Commercialization Model:
- Delivers more value than traditional licensing or launching on your own with a true partnership structure.
- Activates a complete, ready-to-deploy, high-performance commercialization and distribution engine.
- Accelerates your product from early development to marketing, effectively impacting patient outcomes and beyond.
- Provides access to a deep bench of industry experts across all commercial services.
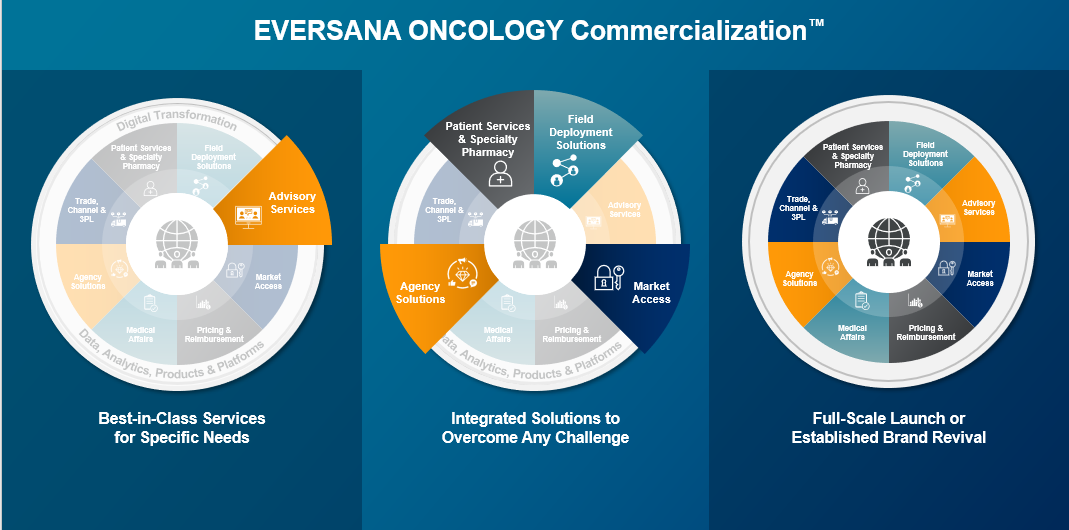
- Meets you where you are on the commercial continuum.
- Supports the entire lifecycle of a product.
- Maximizes and compliments existing internal resources allowing access to new drug candidates and technologies in oncology.
EVERSANA is uniquely positioned to address challenges in the oncology and hematology pipeline with agility and data-driven solutions. With an infrastructure based on product and patient needs, our commercialization model provides manufacturers the flexibility and expertise to customize their strategies and build functional service areas and pull tactics in and out for a successful launch.
With one accountable commercialization partner to make decisions with, manufacturers can enable connectivity between services to manage costs, lower compliance and competitive risks and increase speed to launch in today’s complex market – and ultimately provide timely patient access.
Additionally, the model has the capability to support the entire product lifecycle. By reviving established brands and reigniting revenue, the model aids in mitigating funds lost due to exclusivity and promotes investments in new drug candidates and technologies.
True Financial Benefit
A recent study examined 10 real-world pharma launches and compared them to companies that embraced this new, innovative, scalable commercialization model. The study concluded that those who launched in the traditional model overspent by 23%, without any upside on launch success. Download the white paper to see an in-depth analysis of all launches and costs.
How EVERSANA Serves as an Extension of Your Team: Insights from Shorla Oncology CEO Sharon Cunningham