
Posted on 11 月 18, 202011/18/20
Ensuring Speed, Scale and Certainty in Supply Chain

Posted on 10 月 1, 202010/1/20
Compliance – The Competitive Differentiator to Commercialization: An Integrated Model Setting the New Global Standard

Posted on 9 月 21, 20209/21/20
Navigate the Complexity of Proposed CMS Medicaid Rule
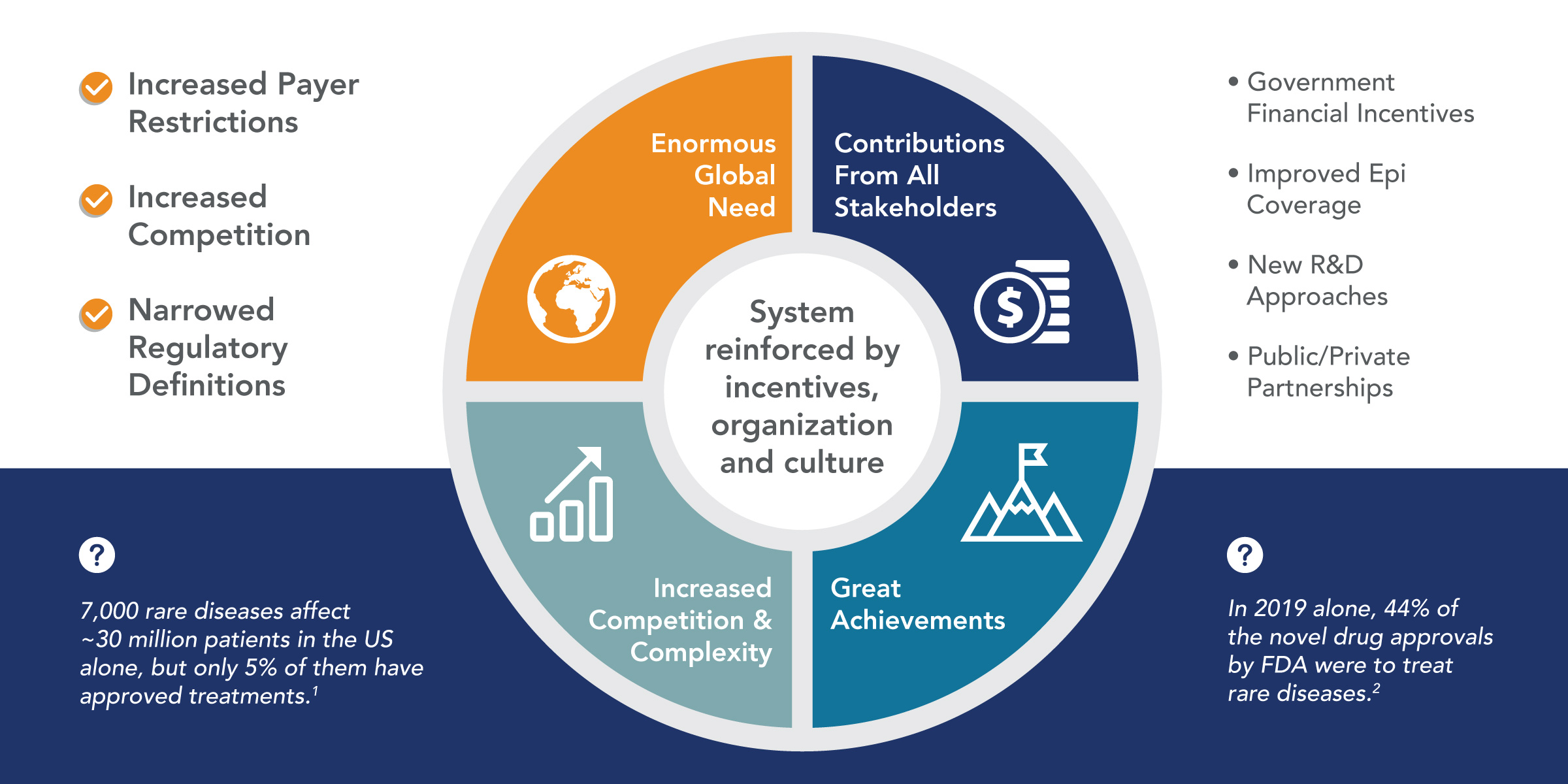
Posted on 9 月 8, 20209/8/20
Doing the Right Things Right: A Perspective on Rare Disease Drug Development

Posted on 9 月 2, 20209/2/20
CMS Announces Medicare Coverage for FDA-Designated Breakthrough Devices. What Does This Mean?

Posted on 8 月 31, 20208/31/20
Looking To Launch Or Commercialise In The US?

Posted on 8 月 14, 20208/14/20
The Regulatory Landscape in Digital Health

Posted on 7 月 16, 20207/16/20
CMS Proposed Medicaid Rule: Best Price Impacts of Value-Based Purchasing, Co-Pay Assistance Programs, and More

Posted on 6 月 24, 20206/24/20
Brand Access Marketplace Dynamics: HIV

Posted on 6 月 24, 20206/24/20

