On August 31, 2020, the Centers for Medicare & Medicaid Services (CMS) issued a proposed rule change that would automatically provide national Medicare coverage for FDA-designated Breakthrough Devices for a four-year period immediately upon FDA approval. This is big news for patients, health care providers and medical device manufacturers.
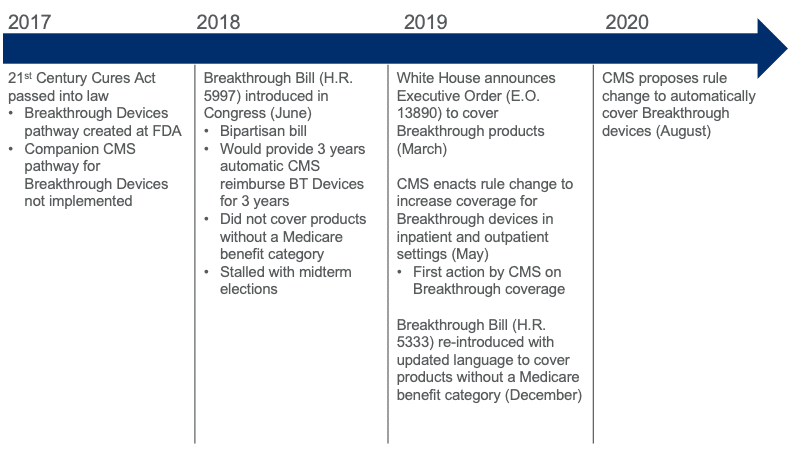
When the Breakthrough Devices program was originally envisioned several years ago, the intent was to “help patients have more timely access” to breakthrough technologies by creating an expedited pathway for both FDA approval and Medicare coverage. The 21st Century Cures Act, enacted in December 2016, initiated the FDA’s Breakthrough Devices Program but did not simultaneously implement a Medicare coverage pathway. This action effectively realizes the original vision and completes the loop.
What Does This Mean for Medical Device Manufacturers?
“For most breakthrough devices, this means that the process to get national coverage is significantly faster and more predictable.”
Even when there is a clear reimbursement pathway for a medical device, it can often take one to two years following FDA market authorization to get coverage; and when coverage is achieved, it may vary from region to region. This proposed CMS rule change effectively eliminates the lag and variability in coverage, thereby expediting and broadening access to Medicare beneficiaries. There is also likely to be a trickle-down effect with private insurers since many private insurers follow Medicare policy.
What This Excludes
However, for breakthrough devices without a traditional reimbursement pathway, the benefits are less clear. Unfortunately, CMS explicitly prohibits breakthrough devices without a Medicare Part A or Part B benefit category from being covered under the proposed rule change. This means that most wearables, digital therapeutics and digital health products would not be covered under this rule change since they do not fit into a benefit category – generally because these kinds of products are so innovative that they did not yet exist when CMS defined how devices would be covered. However, there are some potential opportunities for digital products that have a hardware component that could be considered “durable medical equipment” – a device that can last 3 years and is used daily at home.
In addition, the language is ambiguous as to whether the rule change covers in vitro diagnostics, and it is not likely that products covered by Medicaid are included. Products that are explicitly excluded from Medicare coverage, such as hearing aids, are not covered; and, of course, products that are not devices (such as drugs and biologics) are not included. These decisions were likely made to limit the scope of the new rule change and to contain cost.
What’s Next for Products That Are Not Included?
“The announcement by CMS is a significant step forward for coverage of breakthrough devices. However, there is more work to be done to truly make all breakthrough devices, including some of the most innovative ones, accessible to patients.”
Fortunately, efforts have already been initiated to cover breakthrough devices that do not have a traditional reimbursement pathway. In late 2019, AdvaMed led the introduction of a bipartisan bill (sponsored by Suzan DelBene, D-WA – H.R.5333: “Ensuring Patient Access to Critical Breakthrough Products Act of 2019”) that was very similar to the CMS rule change but also explicitly included language that would have provided coverage for “specified breakthrough devices” that did not have a benefit category. While the proposed CMS rule change obviates this bill, its language has created a framework to cover these kinds of devices.
Additional efforts are underway to create pathways for CMS reimbursement of digital medicine products by AdvaMed through its Digital Technologies Payment Working Group and the Digital Therapeutics Alliance (DTA) through legislative action. A timeline of actions specifically related to breakthrough device reimbursement is provided below.
You can leave a public comment by November 2, 2020, if you want to provide feedback to CMS and suggest that it broaden the inclusion of products beyond those that are identified above. The link to the public comment page for the proposed rule change related to EO 13890 is https://beta.regulations.gov/.
To learn more about Digital Therapeutics, schedule a meeting with one of our Digital Medicine experts.
Author

Marty Culjat, PhD is the SVP, Global Head of Digital Medicine & Regulatory Innovation at EVERSANA. In this role, he leads a cross-functional team supporting the commercialization of digital medicine products within companies ranging…

