NAVLIN Price & Access Data Brochure
Download additional information on the features and benefits of NAVLIN Price & Access Data.
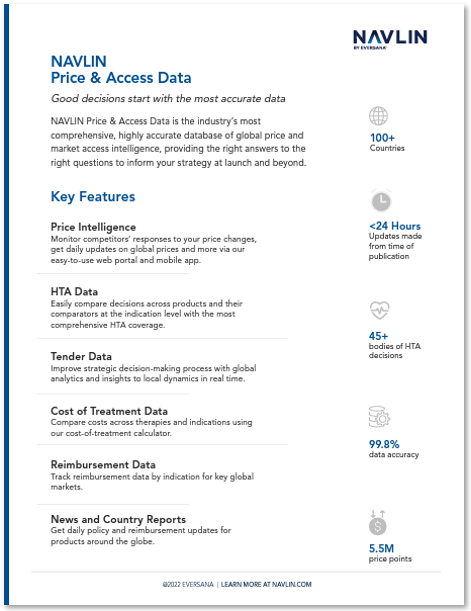
NAVLIN Price & Access Data is the industry’s most comprehensive, highly accurate database of global price and market access intelligence, providing the right answers to the right questions to inform your strategy at launch and beyond.
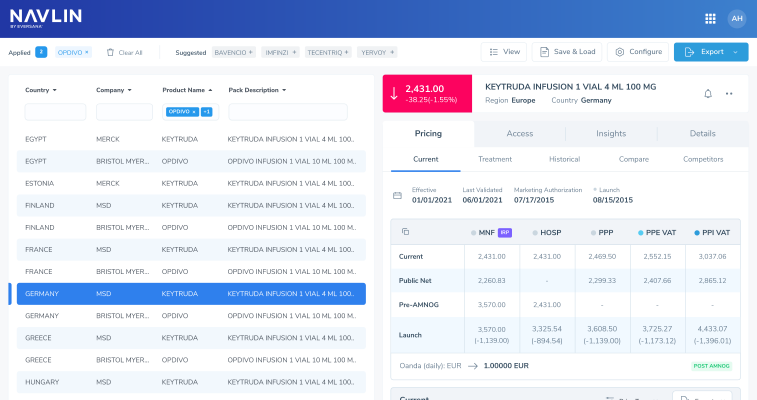
NAVLIN Price & Access Data provides global teams with the data they need in a single consolidated environment.
NAVLIN Price & Access Data is updated in real time, providing users with the industry’s most accurate pricing and market access data. Our database manages every product in more than 100 countries and provinces, including complete historical coverage in most countries.
The combination of comprehensive pricing and market access data, one-click insights and expert support enables teams to focus on strategy and improved decision-making.
Our team of analysts offers 24×7 support and assists in the development of custom reports to meet your most pressing needs.
NAVLIN Price & Access Data provides global pricing, analytics, HTA, regulatory, clinical, and economic data along with news and insights in a single platform.

Download additional information on the features and benefits of NAVLIN Price & Access Data.