
Michael DeLuca
Senior Vice President of Medical Affairs
Expertise:
Mike currently serves as Senior Vice President of Medical affairs at EVERSANA. With 20+ years of healthcare and pharmaceutical industry experience in multiple leadership roles, he holds extensive experience in medical information, medical communications, medical affairs operations, and supporting the medical review of promotional and non-promotional materials. Mike has supported the launch of 10 new products while working at multiple pharmaceutical companies and has expertise across multiple therapeutic areas, including rare disease. Mike is also a Board Member of phactMI.
Articles by Michael DeLuca

Considerations for the Development of Interactive / Innovative Medical Information Content – Insights from a Service Provider
The creation of high-quality, customer-centric medical information (MI) content accessible through various data dissemination channels is crucial. EVERSANA, a provider of global MI contact center services, has embarked on developing more interactive MI content. This initiative involves converting traditional SRDs and FAQs into engaging and innovative formats by identifying considerations essential for this format of MI content. While traditional MI content is valuable, it can be complex and lengthy, prompting this shift towards more navigable and concise formats, particularly for materials with high utilization, high impact, and that will be available via digital channels. Download the poster to further understand the challenge at hand, the solution and the results from […]

Current Trends in Medical Review of Promotional and Non-Promotional Materials
Medical Information (MI) plays a crucial role in addressing inquiries from healthcare professionals, payors, and patients/caregivers and ensuring pharmaceutical products’ safe, effective, and appropriate use. However, MI teams often have other important responsibilities beyond the scope of traditional MI services. EVERSANA conducted a survey in 2023 to assess current trends and to better understand Medical Affairs (MA)/MI teams’ support needs regarding medical review of promotional and non-promotional materials as part of MLR review committees. The online questionnaire was sent to companies that EVERSANA currently provides MI/MA services for, in addition to being shared on LinkedIn by EVERSANA’s MA leadership. A total of 28 pharmaceutical companies responded to the survey, with […]

Considerations for Globalization of Medical Information Services: A Service Partner Perspective
This poster details the considerations for globalizing medical information (MI) services. Traditionally, pharmaceutical companies began in one geographical region before expanding, but evolving regulations and research priorities for rare diseases, oncology/hematology, immunology, and cell and gene therapy have led to simultaneous global regulatory filings. To streamline operations and maintain control, these companies partner with global MI contact center service providers and follow key recommendations. Based on EVERSANA’s experience as a global MI service provider supporting companies moving from product commercialization in only one country/region to multiple regions (either European companies bringing products to U.S. or vice versa), we compiled a robust list of recommended approaches. These include but are not […]

Significance of Promotional and Non-Promotional Materials Review Support Services
Importance of a Well-Defined Medical Legal Regulatory Review Process It is critical for companies to have a well-defined medical, legal and regulatory review process. One of the predominant challenges companies face is that medical and regulatory reviewers often have multiple competing responsibilities. Additionally, the volumes of materials required for review can fluctuate and be significant, especially during new product or indication launch. Having supplementary ongoing and/or temporary support can help organizations better ensure compliance and meet deadlines. Critically Important Role of the Medical Reviewer The Medical Reviewer plays a critically important role in the Medical Legal Review (MLR) process for both promotional and non-promotional material review and approval. The medical […]

Patient-centric Approach to Clinical Trial Support Leveraging Medical Information Services
The number of clinical studies conducted has steadily shown a significant increase year over year for the past two decades. However, the number of new drug and biologic approvals have not seen this same kind of increase. This causes several challenges for manufacturers such as increased competition and difficulty recruiting patients, particularly for rare diseases, cell and gene therapy, oncology, hematology and immunology. EVERSANA’s Medical Information (MI) Contact Center provides clinical trial support services that helps both established and emerging brands combat these challenges by executing a patient-centric approach that improves recruitment rates and brings innovative treatments to the market more efficiently. Patients are able to easily access MI specialists […]
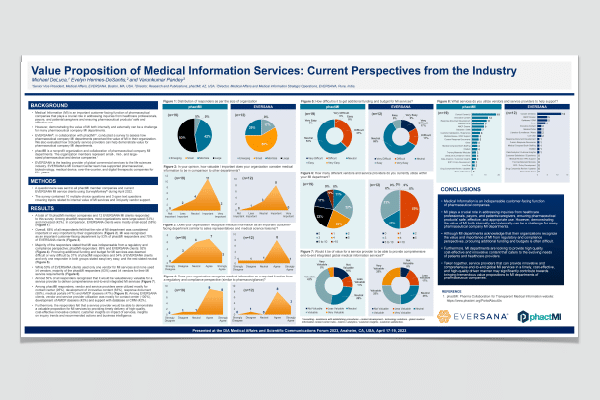
Value Proposition of Medical Information Services: Current Perspectives from the Industry
Medical Information (MI) is an important customer facing function of pharmaceutical companies that plays a crucial role in addressing inquiries from healthcare professionals, payors, and patients and caregivers and ensuring pharmaceutical products’ safe and effective use. However, demonstrating the value of MI both internally and externally can be a challenge for many pharmaceutical company MI departments. EVERSANA®, in collaboration with phactMI™, conducted a survey to assess how pharmaceutical company MI departments perceived the value of MI in their organization. We also evaluated how 3rd‑party service providers can help demonstrate value for pharmaceutical company MI departments. The survey questionnaire prompted respondents to examine the value of MI to their organization, difficulties […]

