PRICENTRIC BRIEF:
- The U.S. Food and Drug Administration (FDA) has announced an official Oncology Drugs Advisory Committee (ODAC) meeting to review six indications across a group of PD-1 and PD-L1 inhibitors that have recently had their indications withdrawn in the U.S. due to an accelerated approvals crackdown
- The panel will assess a group of inhibitors that were granted under the agency’s accelerated approval pathway with confirmatory trials that have not verified clinical benefit, such as Bristol Myers Squibb’s Opdivo (nivolumab), Merck’s Keytruda (pembrolizumab) and Roche’s Tecentriq (atezolizumab) across six different indications in breast, urothelial, gastric and hepatocellular cancers
- The special three-day public hearing on April 27 to 29 will “give oncology experts outside of the FDA and patients with cancer an opportunity to describe their experiences with the drugs,” explained Richard Pazdur, MD, director of FDA’s Oncology Center of Excellence
THE DETAILS
WASHINGTON, D.C., United States – The U.S. Food and Drug Administration (FDA) has announced an official Oncology Drugs Advisory Committee (ODAC) meeting to review six indications across a group of PD-1 and PD-L1 inhibitors that have recently had their indications withdrawn in the U.S. due to an accelerated approvals crackdown.
The panel will assess a group of inhibitors that were granted under the agency’s accelerated approval pathway with confirmatory trials that have not verified clinical benefit, such as Bristol Myers Squibb’s Opdivo (nivolumab), Merck’s Keytruda (pembrolizumab) and Roche’s Tecentriq (atezolizumab) across six different indications in breast, urothelial, gastric and hepatocellular cancers.
Richard Pazdur, MD, director of FDA’s Oncology Center of Excellence, noted that the committee is “committed to ensuring the integrity of the accelerated approval program, which is designed to bring safe and effective drugs to patients with unmet medical needs as quickly as possible.
“The program allows the FDA to approve a drug or biologic product intended to treat a serious or life-threatening condition based on an outcome that can be measured earlier than survival that demonstrates a meaningful advantage over available therapies. However, when confirmatory trials do not confirm clinical benefit, a reevaluation must be performed to determine if the approval should be withdrawn.”
Oncology drugs and indications up for review:
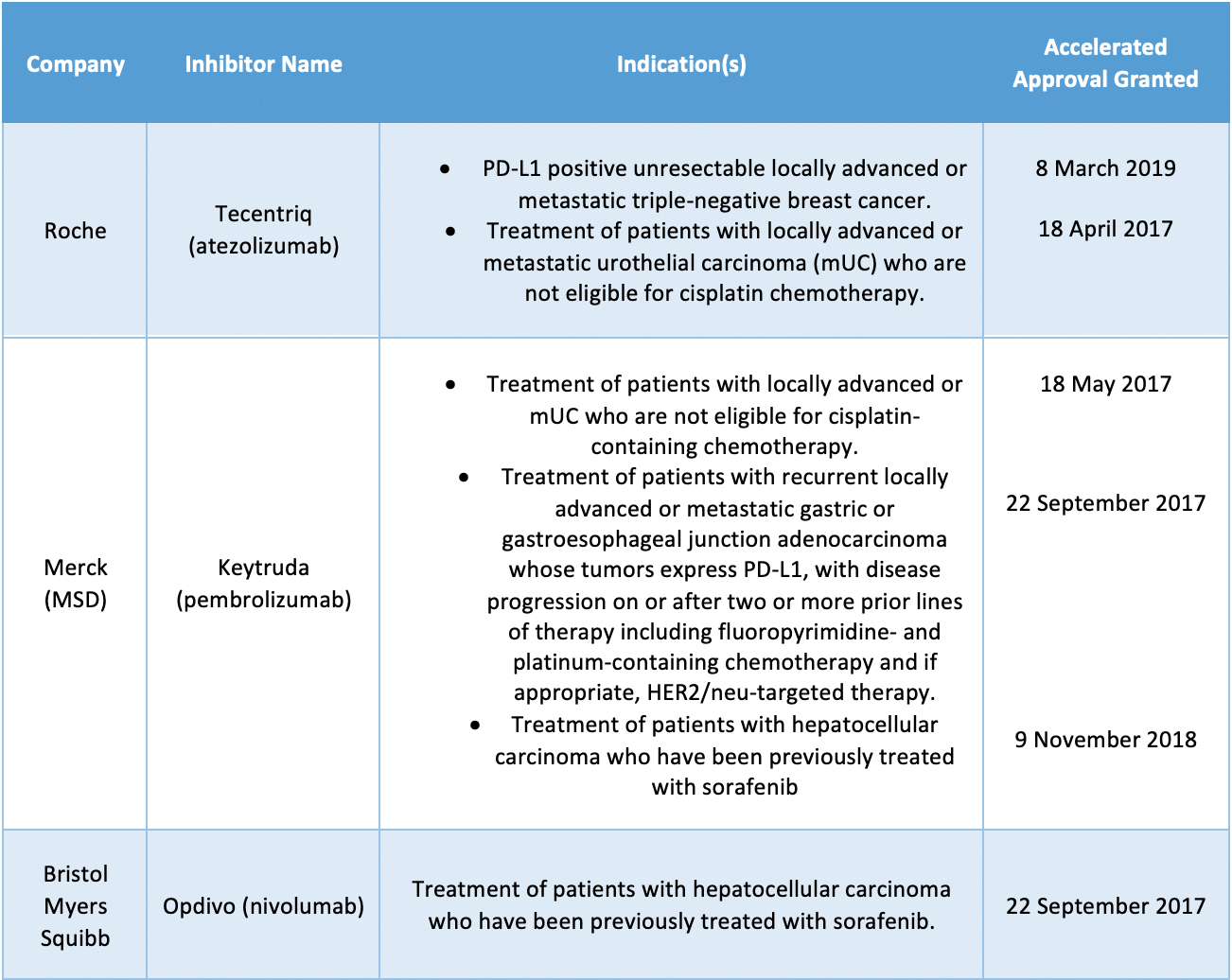
Genentech recently announced that it was voluntarily withdrawing the U.S. indication for Tecentriq in prior-platinum treated metastatic urothelial carcinoma, in a joint decision made in consultation with the FDA.
Merck also voluntarily withdrew its U.S. indication for Keytruda for patients with metastatic small-cell lung cancer (SCLC) who experience disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy earlier in March, and in December 2020, Bristol Myers Squibb also announced its withdrawal from the FDA of an application regarding Opdivo (nivolumab) in SCLC.
BMS issued a note of support for the FDA’s decision, stating that “In HCC, despite evolution of the treatment landscape over the past few years, we believe Opdivo continues to address an unmet medical need for patients in the post-sorafenib setting, and we appreciate the opportunity to discuss this in more depth with the Committee.”
The special three-day public hearing on April 27 to 29 will “give oncology experts outside of the FDA and patients with cancer an opportunity to describe their experiences with the drugs,” explained Pazdur.
According to the FDA, only 6% of accelerated approvals for oncology drugs have been withdrawn over the whole duration of the pathway’s use, including the four recent withdrawals.
Learn more about Pricentric ONE and our Global Pricing Solutions!
Contact us with your questions and global pricing needs, and an expert will follow up shortly.

