Insights
Insights From Our Experts
Articles

NEXT GENERATION COMMERCIALIZATION: A CONVERSATION WITH THE INNOVATORS
Sell, out-license or launch internally: these are the three traditional options to commercialize a pharmaceutical product. Selling and out-licensing are common but cause innovators to lose ownership in an investment that takes years to develop. Launching…

WODC 2020 Insights: Leveraging AI/ML to Advance Rare Diseases
Leveling the playing field The average time to correctly diagnose a patient with a rare disease is 7 years, largely due to data sparsity, misdiagnoses, incorrect prescriptions, etc. Now, through the application of artificial intelligence and…

Navigate the Complexity of Proposed CMS Medicaid Rule
In this presentation at MDRP’s virtual summit, Mike Kurland discusses several key regulatory proposals and executive orders and the impact on pharma: Operationalizing Value-Based Contracting – Has the door been opened, and how do we prepare…

At the COVID-19 Finish Line, How Do We Price The Winning Vaccine?
As of August 21, 2020, there were more than 165 potential vaccine candidates in development for the novel coronavirus. While some are still in preclinical stages, a handful are quickly making their way toward regulatory approval,…

How to Get Your Digital Therapeutic Approved & Reimbursed in Europe and the U.S.
Patients, providers, and payers expect healthcare to be more accessible, intuitive, and adaptable to their needs. Barriers that have held back innovation in Digital Medicine and Telemedicine have been blasted apart with the recent relaxing of…

PharmaVOICE100 Recognizes Two EVERSANA Leaders
For the past 15 years, PharmaVOICE has celebrated leaders throughout the life sciences industry who provide inspiration to their peers, colleagues and companies through their innovative and motivational approaches to addressing the industry’s myriad challenges. As…
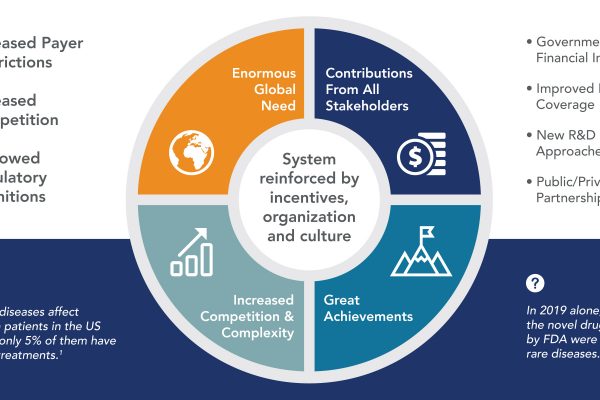
Doing the Right Things Right: A Perspective on Rare Disease Drug Development
Millions of patients with rare diseases have benefited from the emphasis biopharmaceutical companies have put on the development of orphan drugs over the last decades. The more patients served, the more complex and challenging it becomes,…

WODC 2020 Insights: Innovative Value and Access Strategies
Innovative rare disease therapies deserve innovative value and access strategies. With over 1,500 attendees from 50+ countries, the World Orphan Drug Congress featured four days of education, conversation and brainstorming dedicated to drug development and commercialization.…

CMS Announces Medicare Coverage for FDA-Designated Breakthrough Devices. What Does This Mean?
On August 31, 2020, the Centers for Medicare & Medicaid Services (CMS) issued a proposed rule change that would automatically provide national Medicare coverage for FDA-designated Breakthrough Devices for a four-year period immediately upon FDA approval.…

Looking To Launch Or Commercialise In The US?
Launching a product is a serious undertaking, requiring a seamless approach to building a commercialisation strategy that includes all the variables needed to maximise the investment across the product’s lifecycle. Nowhere is this more true than…

