
Posted on February 5, 20242/5/24
NAVLIN Insights Special Report: Three Mergers and Acquisitions Changing the Health Insurance Landscape for 2024

Posted on October 19, 202310/19/23
NAVLIN Insights Sneak Peek: Brand Access Landscape, Multiple Sclerosis Now Available

Posted on October 17, 202310/17/23
NAVLIN Insights Sneak Peek: 340B for Specialty Pharmacies 2.0 Now Available
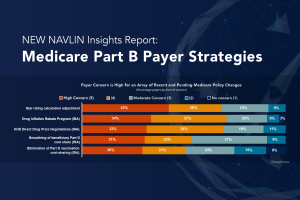
Posted on October 17, 202310/17/23
NAVLIN Insights Sneak Peek: Medicare Part B Payer Strategies Now Available

Posted on August 31, 20238/31/23
Webinar: Empowering HTA in Europe: Unleashing the Potential of ITC, ECA & PROM Methods to Improve Access

Posted on August 31, 20238/31/23
NAVLIN Insights Special Report: Department of Health and Human Services Releases Intended Targets for Medicare Price Negotiations

Posted on August 24, 20238/24/23
NAVLIN Insights Special Report: Blue Shield of California Moves Against the Big 3 PBMs
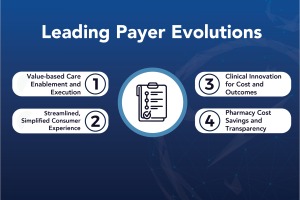
Posted on August 16, 20238/16/23
NAVLIN Insights Sneak Peek: Channel Access – Leading Payer Evolution Report Now Available

Posted on July 18, 20237/18/23
NAVLIN Insights Sneak Peek: New Patient Access – Biosimilar Boom Research Now Available
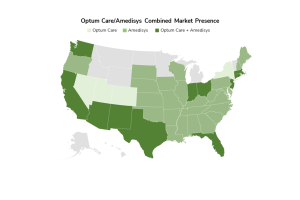
Posted on July 7, 20237/7/23

